January 2022
Editor introduction
This Rhaïa edition provides insight into cancer, spinal cord injury (SCI), and geriatric rehabilitation. Firstly, it is pleasing to see a lot more activity and growing interest amongst our colleagues, especially our younger Fellows, in the cancer rehabilitation space. Cancer patients are increasingly needing access to rehabilitation care and interventions. A recent exploratory pilot survey of rehabilitation colleagues working in cancer rehabilitation in Australia and Aotearoa New Zealand identified inadequate funding, lack of staff with expertise, and lack of collaboration between acute cancer care services and rehabilitation as major barriers towards successful implementation of cancer rehabilitation programs.1
The hope is to gradually dismantle these barriers, support a strategic approach to ensuring that cancer rehabilitation programs are available in our region, and develop sustainable, holistic cancer care models. Our cancer Special Interest Group (SIG), relevant working groups, and interested rehabilitation Fellows will play a vital role in promoting the awareness of cancer rehabilitation amongst acute specialty teams and community care providers. They will contribute to capacity building, establishing clinical guidelines, policies and pathways, encouraging research activities, and increasing advocacy of this area for the future.
With SCI rehabilitation, the latest SCI data from the Australian Spinal Cord Injury Register (ASCIR) showed 187 new cases of adult traumatic SCI in 2017-2018.2 Similar to previous findings, the majority were of younger age (25-34 years old), male gender, had neurological level of injury at C4 and incomplete tetraplegia (43 per cent) at discharge. Land transport crashes (46 per cent) represented the leading cause, followed by falls (36 per cent). For non-traumatic SCI (vascular disorders, degenerative conditions, cancer), out of 131 cases, males accounted for 60 per cent and the mean age was 58 years (range 19-89 years).
As we are aware, the short- and long-term disabilities faced by SCI patients are multiple. Useful resources can be found on SCI organisational websites (AQA Victoria, ParaQuad Tas, ParaQuad NSW, Spine & Limb Foundation WA, SCIA, SpinalCure, Spinal Life) and through societies such as ANZSCoS and ISCoS. Interesting research trials being undertaken include the eWalk (transcutaneous neurostimulation for SCI, Sydney) and robotic assisted game-based therapy.
We are also facing a population with increasing life expectancy. The need for ‘geriatric rehabilitation’ is growing in our region and so is the need for rehabilitation physicians with the essential skills to manage issues in older people. These can include sensory impairments (vision, hearing), falls, osteoporosis, malnutrition, depression, and cognitive impairment, including delirium and dementia. Professor Ian Cameron flags this area as a critical topic and Professor Mary Galea discusses osteosarcopenia.
I would like to express my utmost appreciation to all of our writers for their contribution to this edition of Rhaïa especially during a very busy and challenging year. If you have any interesting rehabilitation news that you would like to share with your colleagues, please let us know and we can include it in future publications. I wish you all the very best and I am sure that we are all looking forward to new and exciting things ahead in 2022.
Dr Krystal Song
Editor
References:
- Song K, Amatya B, Khan F. Cancer rehabilitation in Australia and New Zealand: a pilot cross-sectional survey. The Journal of The International Society of Physical and Rehabilitation Medicine 2021; 4(3): 146-155.
- Australian Institute of Health and Welfare: Harrison J, O’Brien D, Pointer S. Spinal cord injury, Australia, 2017–18. Injury research and statistics series no. 136. Cat. no. INJCAT 219. 2021. Canberra: AIHW.
Readers, please note that the below articles were written several months ago. Given the fast changing circumstances of the COVID-19 pandemic, some information may have changed since the articles were originally written, particularly in relation to the COVID-19 situation in Victoria.
President’s Report
I congratulate Editor Krystal Song for drawing together three very important areas of rehabilitation practice: cancer rehabilitation, spinal cord injury, and geriatric rehabilitation in this edition of Rhaïa.
I hope all three areas are now seen as core business by rehab medicine departments, but it was not always thus. They remind us how things change. Spinal Injury units were first established many decades ago in response to the number of young patients who were catastrophically injured in accidents, and whose prognoses were dire without specialist knowledge and treatment. The demographics have changed greatly during my career from a predominantly young person’s injury to one which is now seen almost as frequently in a much older population, related to falls.
In the early part of my career, cancer treatment was not something that involved rehabilitation services much at all. Fortunately, this has changed in the past 20 years and much of the credit is due to passionate advocates such as Professor Andrew Cole, who has contributed to this edition. Geriatric rehabilitation remains a very important part of practice for many of us and an important interface between us and our geriatric medicine colleagues in many of our hospitals and community practices.
Our colleague, Dr David Bowers, passed away recently and unexpectedly in the full flight of his professional career. Many of you would have known him, a spinal injury rehabilitation physician at the Royal North Shore Hospital in Sydney. Fitting tributes to his care and compassion were expressed on behalf of his colleagues and patients at his funeral service which was, of course, greatly limited in scope due to COVID-19 restrictions. On behalf of the Faculty, we would like to convey our sincere condolences to his wife and children.
Work at the Faculty continues on many fronts in a COVID restricted way, just as you are experiencing in your own hospitals and practices. We continue to meet regularly at executive and council level but these are all virtual meetings now. The unpredictable nature of the pandemic continues to frustrate attempts to normalise events. My recent plan to attend the New Zealand Rehabilitation Association meeting during the travel bubble and when things were more settled evaporated very rapidly and now appears hopelessly optimistic in retrospect. You probably have started attending virtual international meetings as I have. Whilst I find the convenience of not having to travel very helpful, I have had to confront the reality that these sessions are generally live in the middle of the night here, which tends to be rather disorienting if not overpoweringly soporific.
All the Faculty Examinations for 2021 have concluded, including the delayed Victorian Module 2 Examination in Melbourne, which was held on October 10th. Clearly, the COVID situation in Sydney and Melbourne were of concern and there was a contingency plan to delay until November if needed. We have started preparations for our 2022 examinations. The landscape for next year is not clear yet, although with vaccination rates at the level that they are, we are hopeful that there may be more normality by that time. Meetings of the Examination Working Group, comprised of the Faculty Executive, Chair of Assessment Committee and College education staff have been a regular feature of the last 12 months.
We are progressing our plans for a greater academic presence by virtue of a permanent academic post on the Faculty Council, which is underway and will link to a permanent research committee of Council. We continue to meet regularly with the Australian Rehabilitation Outcomes Centre (AROC), represented by the Director of UOW Health Services Research Institute Kathy Eagar, newly appointed Medical Director of AROC Maria Crotty, and recently appointed Director of AROC Ross Clifton. AROC is currently undergoing a strategic review, seeking to use its reputation as the best health benchmarking system in Australasia to seek ways where that data can be used to influence health policy at a state and national level. We are currently still seeking a Chair of the Management Advisory Group to replace Dr John Estell whose term finishes soon.
The place of rehabilitation in COVID-19 continues to be a very important topic. Dr Susan Graham represents the Faculty on the College’s COVID-19 Expert Reference Group and was recently involved in organising webinars on Long COVID. We remain concerned at reports of reduction in rehabilitation services and resources throughout the country in response to the need to expand acute services, particularly in Sydney recently and in Melbourne last year. Our concern is that these resources are often not restored or alternative proposals seem to appear almost under the guise of COVID. The literature suggests that there will be significant need for rehabilitation services for so called Long COVID and we need to be mindful that this may be the opportunity to ensure that rehabilitation services are not allowed to be permanently reduced and marginalised.
The College launched the ROC (RACP online communities) with the official launch of the Faculty community in October. I urge all members to join the online community. We hope that it will provide an easy method of communication between members, which will avoid the previous intractable problems when lists of email addresses were not available. We hope to be able to set up much easier direct communication for Branch Committees and Special Interest Groups.
There are always vacancies occurring in Faculty Committees, all the way up to executive and we are constantly looking to identify the next generation of Fellows to take over the important roles at the head of many of our committees. We need long-term successors for the Chairs of the Faculty Assessment and Faculty Training Committees and the President-elect becomes available in May 2022. I ask that all New Fellows consider where you could help and where your interest may lie. Those who have been working hard on committees already are requested to seriously consider moving to the Chair of the committee if you can. Committee members are often in the best position to take over the chair as they have a good understanding of how the committee works. It is often more difficult for someone to step in as the Chair of a committee when they have not been involved within before.
Rural colleagues have recently raised the issue of lack of continuity of trainees working in rural services and the difficulties this raises for their services from time to time. The rural workforce is a very big issue at both government and College level. The maldistribution of our health workforce is well documented and is indeed part of the problem with Indigenous health. Dr Jeremy Christley is the Faculty representative on the College’s Regional and Rural Physician Working Group. Rural training is a complex issue and one which the Faculty considered formally about 10 years ago, although the focus was about attracting Fellows to work in the country.
Trainees who have worked in the country are more likely to consider a rural career as a realistic option. I have had recent talks with the Australian College of Rural and Remote Medicine and the Rural Health Alliance both of whom understand the issue of convincing health professionals to see a rural career as a realistic medium- or long-term option. This is a complex issue and needs consideration of both the projected long-term rural workforce, the quality of the training experience, and the burden, both socially and financially, on trainees particularly now that many of our trainees are older than a generation ago and their life can be correspondingly more complicated. I am hopeful that if we can delineate and try to address the economic and social disincentives where they exist and simultaneously promote the value of rural training, this will allow us a non-controversial path to increase the number of trainees taking up country positions.
Please continue to make yourself aware of our College and Faculty prizes available every year. Please take the time to look at the College website and consider your colleagues who might be appropriate to nominate for these medals. There are several scholarships for those wishing to undertake research, with some specifically reserved for AFRM trainees and Fellows and others available to the wider College membership. Please promote these to your trainees and New Fellows who may be interested in a career in research.
A considerable amount of time was spent during the last two to three years developing materials to support an increase in the profile of rehabilitation medicine. These include PowerPoint presentations, brochures, and letters of introduction that can be used by Fellows to inform employers, area health service executives, and other health stakeholders of the value of rehabilitation medicine and its contribution to the health landscape. Unfortunately, much of this effort has been swamped by the pandemic that essentially sweeps all before it, but we have continued to meet with stakeholders to help them understand more about who we are and what we do. I have found these meetings to be beneficial, making personal links to partner organisations when the time is appropriate.
I continue to be very grateful for the support and assistance of my fellow Executive Members Jenny Mann (President-elect), Tim Geraghty (immediate past President), Caitlin Anderson (Chair, Faculty Education Committee), and Richard Seeman (Chair, Aotearoa New Zealand Branch), as well as our College staff, Jane Henderson (Executive Officer), Jo Goldrick (Faculties Manager) and Phil Munro (Peak Bodies Manager). I wish you all the very best in these trying times and we look forward to when the coming and expected COVID surges in New South Wales and Victorian hospitals have passed their peaks.
Dr Greg Bowring
AFRM President
Cancer rehabilitation
Until recently, cancer survivors have comprised only a small part of most rehabilitation physicians’ case load. Although some cancers have long been cured with early treatment, recent advances in chemotherapy, radiation therapy and immunotherapy now mean that treatment of quite advanced metastatic disease can lead to prolonged patient survival, for example with melanoma, breast, and prostate cancer. This, however, may be associated with significant impairment and functional disability. In this century, cancer has become another chronic disease, and survivors will respond to rehabilitation programs, just as others living with more ‘benign’ diseases do.
Rehabilitation helps cancer patients maximise their functional abilities and quality of life at each stage in their journey, as first recognised by Dietz in 1969. Programs start with preventive measures from the time of first diagnosis, later using restorative measures to regain premorbid functions lost with cancer treatment or combined with the effects of frailty in older cancer survivors. If significant physical problems are present (e.g., neurological or musculoskeletal impairments after treatment of cancer), intensive goal-orientated multidisciplinary programs are of great help. Finally, even in the later stages of the journey, relatively simple rehabilitation interventions provided jointly with palliative care clinicians can maximise residual mobility, activities of daily living or continence function, and greatly improve quality of life for both patients and their carers.1
Oncology care clinicians may not be aware of good outcomes from rehabilitation, may be focused on disease management rather than functional decline, or may wrongly think that if cancer-modifying treatment is of no further use, then rehabilitation also has little to offer. Likewise, cancer survivors and carers may assume and simply accept that progressive functional loss is inevitable with disease progression. All these are barriers to cancer survivors receiving appropriate access to rehabilitation.
Apart from major physical impairments, uncontrolled pain with depression, decreased activity and deconditioning are major causes of lost functional ability. It is important that baseline rest pain is properly controlled, with extra analgesics prescribed prior to general activities likely to provoke episodic pain (e.g., physiotherapy). Other activities that cause pain can be carefully deconstructed, to identify the specific motion or position that triggers pain, which can then be dealt with.
Both frailty and cancer are not uncommon in older people, and presence of frailty may well predate a cancer diagnosis. With early recognition, the older cancer survivor can be moved towards a non-frail state if given a combined program of nutritional support and physical exercise. This is especially important if there is complicating nausea or anorexia associated with chemotherapy or radiation therapy programs. At any age, if deconditioning is present in a cancer survivor, they should be offered an appropriate rehabilitation program for this, especially if their goals in life recovery include a return to work.
For cancer survivors with neurological deficits due to primary or metastatic brain or spinal cord lesions, and where the underlying malignant disease process has been treated and stabilised, there is good evidence that they respond to rehabilitation therapy with similar functional recovery, as do patients recovering from strokes or spinal cord ‘benign’ lesions, matched for anatomical location. This applies to therapy programs in individuals with speech and/or cognitive involvement, as well as with peripheral neurological involvement, whether from chemotherapy or individual peripheral nerve impingement by tumours.
It is particularly important that cancer survivors with brain stem disease are offered detailed speech therapy, occupational therapy, nutritional and swallowing programs, with appropriate dietary modifications or alternative feeding approaches, to avoid chest infections and frustrations with dysarthric speech and visual/coordination problems.
As with all rehabilitation, training and support of carers is most important, to maximise quality of life at home for the cancer survivor and their family/carers.
There is no place for therapeutic nihilism in the rehabilitation of a cancer survivor!
Associate Professor Andrew M Cole FAFRM
HammondCare Health, Greenwich Hospital
UNSW, School of Population Health NSW
References:
Cole AM. Medical rehabilitation and the palliative care patient. In: Cherny NI, Fallon MT, Kaasa S, Portenoy RK, Currow DC, editors. Oxford Textbook of Palliative Medicine. 6th ed. Oxford, England: Oxford University Press; 2021. P.255-264.
An update on cancer pain
Cancer rehabilitation is increasingly recognised as a crucial component of care for cancer survivors, with rehabilitation clinicians playing an integral role in supporting cancer patients during most phases of their cancer trajectory.1,2 Cancer pain is one of the most common symptoms experienced by cancer patients, with potential occurrence in any cancer type and at any stage from diagnosis to end-of-life care.3 Cancer pain can be classified as acute or chronic pain, and is defined as ‘pain that arises from cancer itself and/or treatment-related complication(s)’.4 If not managed well, cancer pain can have a significant impact on patients’ physical and psychological functions, resulting in difficulties carrying out their day-to-day activities and community roles.
Cancer pain is a complex condition that involves various pathophysiological mechanisms. Common cancer pain syndromes include chemotherapy-induced peripheral neuropathy (CIPN), pathophysiological fractures, widespread pain syndromes and persistent post-surgical pain.5 Contributing factors include nociceptive pain from surgery, radiation therapy, and cancer itself, from invasion of cancer into tissue or bone.
Peripheral and central sensitisation from persistent inflammation and repeated nociceptive input from cancer treatment and investigations may also contribute to persistent cancer pain.6 Many factors influence the experience of cancer pain including the patient’s background, experiences, cognitive status, and perpetuating biopsychosocial factors such as dealing with the notion of death, fear of cancer recurrence, and traumatic experiences from cancer treatments.7 Therefore, a thorough assessment of cancer pain is essential in understanding the mechanism of pain, patient factors, prognostic factors, and expectations of pain management to help guide appropriate treatment.
To date, there is limited evidence for the most effective treatment for cancer pain. Overall, a multidisciplinary approach to pain management is recommended due to the multi-faceted nature of cancer pain and associated symptoms that co-occur such as fatigue, anxiety and/or depression. Pharmacologically, opioids remain the mainstay of treatment for cancer pain but there is increasing awareness of the detrimental long-term effects of opioid use, especially in those with chronic pain syndromes. Side effects include constipation, cognitive impairment, endocrine disorders, and dependence.
Anti-neuropathic agents commonly used in cancer pain include anticonvulsants and antidepressants, but their efficacy is mixed and limited in evidence. Other adjuncts include steroids, alpha-agonists, and anti-inflammatories. Medicinal cannabis is emerging as an option but there is lack of evidence for its use in cancer pain, with implications of cost and tolerability in Australia.8 Non-pharmacological strategies and interventional techniques may be effective in opioid-unresponsive pain and should be considered to reduce polypharmacy and its effects. Nerve blocks, such as epidural injections, can be considered if there is an anatomical target or nociceptive driver. For those with opioid-unresponsive pain or side effects, an intrathecal opioid pump could be considered.
In conclusion, due to the complexity of cancer pain and its management, current evidence supports a more holistic approach to the management of cancer pain, with beneficial effects shown for rehabilitation programs incorporating multimodal interventions such as exercise and psychological strategies.
Dr Jamie Young MBBS, FAFRM, FFPMANZCA, Clin Dip Pall Care, AFRACMA, PhD
Pain & Rehabilitation Specialist
Peter MacCallum Cancer Centre VIC
References:
- Lisy K, Denehy L, Chan R, Khan F, Piper A, Jefford M. The state of cancer rehabilitation in Australia. Journal of Cancer Rehabilitation 2018; 1:9-13.
- Chowdhury RA, Brennan FP, Gardiner MD. Cancer rehabilitation and palliative care - Exploring the synergies. Journal of Pain and Symptom Management 2020; 60(6): 1239-1252.
- Van Den Beuken-Van MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. Journal of Pain and Symptom Management 2016; 51(6):1070-90.
- Yoong J, Poon P. Principles of cancer pain management: An overview and focus on pharmacological and interventional strategies. Australian Journal of General Practice 2018; 47(11):758-62.
- Caraceni A, Weinstein SM. Classification of cancer pain syndromes. Oncology 2001; 15(12):1627-40.
- Falk S, Bannister K, Dickenson AH. Cancer pain physiology. British Journal of Pain 2014; 8(4):154-62.
- Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. Journal of Pain and Symptom Management 2002. 24(5):526-42.
- Lintzeris N, Driels J, Elias N, Arnold JC, McGregor IS, Allsop DJ. Medicinal cannabis in Australia, 2016: the cannabis as medicine survey (CAMS‐16). Medical Journal of Australia 2018; 209(5):211-6.
Breast cancer rehabilitation: Current challenges
It is estimated that over 19,000 Australian women were diagnosed with breast cancer in 2020. This is a startling statistic, but encouragingly, over 90 per cent will survive at least five years.1 During my time seeing women for rehabilitation who have had a recent diagnosis of breast cancer, I have been in awe of their overwhelming positivity, future-thinking approach, and their remarkable ability to continue being mother, daughter, wife, friend and work colleague during what will be one of the most challenging and demanding times of their lives. For this very reason, breast cancer rehabilitation needs to be multi-faceted. It needs to assist women physically, emotionally, and practically to return to these roles which give their lives meaning and purpose.
The list of potential complications following breast cancer treatment is long. Physical effects, which are not necessarily any more or less burdensome than many of the emotional and psychological challenges, include lymphoedema, nerve palsies, chemotherapy-induced peripheral neuropathy, joint pains, fatigue, hot flushes, cording, adhesive capsulitis, osteoporosis, treatment related cardiac toxicity, and cancer-related fatigue. As rehabilitation physicians, we are uniquely placed to assist in the treatment of every single one of these issues. Yet, unless patients are referred to us, our skills go unused and patients miss out on prevention of and early intervention for cancer and treatment related complications.
As an example, a study by Younus et.al (2010) reported that 83 per cent of patients on aromatase inhibitors (AI) described joint-related pain, but only six per cent were referred to rehabilitation.2 Yet, the first line treatment for AI related joint pain is exercise. A breast cancer diagnosis should go hand-in-hand with a referral to rehabilitation, since increasing evidence is available for the benefits of exercise pre-surgery and whilst undergoing chemo- and radiation therapy treatment. At present, it is common for acute cancer care services to make a referral to rehabilitation once treatment is close to completion. However, a proactive approach to rehabilitation involving referral at time of diagnosis can help identify current issues and anticipate future impairments that may occur as a consequence of treatment or disease progression. Increasing awareness of the benefits of early rehabilitation amongst the community is also key; patients are powerful in initiating their own referrals. If patients knew that exercise improves survival and reduces all cause and breast cancer mortality, wouldn’t more patients ask their oncologists or breast surgeons for a rehabilitation referral? Surely the answer is, yes!
It is imperative that cancer rehabilitation programs are tailored to disease stage. A one-size-fits-all model for breast cancer fails to consider the wide-ranging differences in physical and psychosocial needs between those women with early-stage versus metastatic disease. Caution is required with exercise in those with metastatic bone disease due to risks of pathological fracture and spinal cord compression. MacMillan Cancer Support guidelines recommend that Mirel’s classification is used to determine relative fracture risk and therefore the potential need for prophylactic surgical treatment before exercise is undertaken.3
Mirel’s classification (Table 1) quantifies fracture risk based on site, pain, extent of cortical involvement and whether the lesion is lytic, blastic or mixed, and can be calculated from plain x-ray. Objective assessment of fracture risk allows exercise programs to be tailored to the individual patient to reduce potential complications and target safe exercises which still maximise physical outcomes. Activity for those with metastatic bone disease should avoid excessive torsion and reduce shear and compressive forces on affected skeletal sites. Metastases causing functional bone pain, especially in the lower limbs, may be suitable for prophylactic fixation prior to commencement of an exercise program (Table 2). Surgery for these patients has been shown to increase mobility and improve pain, thereby optimising function and improved quality of life.
Table 1: Mirel’s classification3
|
|
Score
|
|
|
1 point
|
2 points
|
3 points
|
|
Site
|
Upper limb
|
Lower limb
|
Peritrochanteric area of femur
|
|
Pain
|
Mild
|
Moderate
|
Functional impairment
|
|
Lesion
|
Blastic
|
Mixed
|
Lytic
|
|
Lesion size
|
<1/3 bone cortex
|
1/3 - 2/3 bone cortex
|
>2/3 bone cortex
|
Table 2: Mirel’s Clinical Recommendations3
|
Mirel’s Score
|
Clinical Recommendation
|
|
≤7
|
Radiotherapy and observation
|
|
8
|
Use clinical judgement
|
|
≥9
|
Prophylactic fixation
|
Further, there is a need to break down the socio-economic divide in provision of breast cancer rehabilitation. It is well documented that those with lower levels of education and income are less often referred to rehabilitation, have more advanced cancer at time of diagnosis, and have greater levels of comorbidity; all of which contribute to poorer outcomes.4 There needs to be improved accessibility to timely rehabilitation interventions for cancer patients across various cancer streams within the public health system in Australia and more funding dedicated to ‘prehab’ cancer services. Prehab for cancer patients can lessen the potential cardiotoxic effects of chemotherapy, improve tolerance to chemotherapy through systemic conditioning, prevent sarcopenia, and target specific body regions susceptible to disease or treatment-related dysfunction.
There is a long way to go before we can confidently say that there are adequate rehabilitation services for the over 70,000 women living with breast cancer in this country. However, the grass roots of current breast cancer rehabilitation programs demonstrate encouraging results and hopeful prospects for further growth. Let’s all raise awareness of our skill set in this area and continue to talk more about cancer rehabilitation, both within our specialty and outside of the rehabilitation sphere.
Dr Faye Jansen
Rehabilitation Physician, FAFRM
Cabrini Health Elsternwick, VIC
References:
- Australian Institute of Health and Welfare. Cancer data in Australia 2021 report. [Accessed 4/8/2021]. Website: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary
- Younus J, Kligman L. Management of aromatase inhibitor–induced arthralgia. Curr Oncol 2010; 17(1):87–90.
- Physical activity for people with metastatic bone disease: Guidance for healthcare professionals. Macmillan Cancer Support 2020. [Accessed 4/8/2021]. Website: https://www.macmillan.org.uk/healthcare-professionals/news-and-resources/guides/physical-activity-for-people-with-metastatic-bone-disease
- Bradshaw PT, Ibrahim JG, Khankari N, et al. Post-diagnosis physical activity and survival after breast cancer diagnosis: the Long Island Breast Cancer Study. Breast Cancer Res Treat 2014; 145:735–42.
Epworth Health cancer rehabilitation initiatives
Depending on cancer type, cancer effects and cancer-related treatments received, cancer patients may present with various rehabilitation issues including fatigue, pain, lymphoedema, chemotherapy induced peripheral neuropathy, steroid myopathy, deconditioning, and malnutrition. They often experience associated psychological distress and reduced overall quality of life. Cancer rehabilitation is increasingly recognised as a strategy to optimise the functional outcomes of cancer survivors from the time of diagnosis and throughout the trajectory of cancer continuum including treatment, survivorship, and advanced stages.
Current evidence supports cancer rehabilitation interventions in helping reduce symptom burden and improve quality of life amongst cancer patients. Evidence has also shown that exercise itself is beneficial in improving aerobic fitness, physical functioning, muscle strength, health-related quality of life, and reducing fatigue levels amongst patients with various cancer diagnoses.
Rehabilitation physicians play an important role in acute, subacute-inpatient, and subacute-outpatient care settings in being able to offer expertise in functional assessment and helping address impairments and activity limitations of cancer patients. At Epworth Healthcare in Victoria, a number of recent initiatives had been implemented to meet the rehabilitation needs of cancer patients across the care continuum. Of note, an ‘in-reach’ rehabilitation physician consult service was established this year within the acute haematology ward at Epworth Freemasons.
The rehabilitation physician assesses referred patients for physical and functional issues impacting on acute recovery and progress, working closely with acute therapists in setting achievable short-term goals. For patients who are not ready to participate in three hours of uninterrupted therapy per day (usual criteria for transfer to an inpatient rehabilitation unit) or are impacted by fatigue, this approach allows rehabilitation to commence in an acute setting for cancer patients. The rehabilitation physician also supports the nurse assessors in assessing suitability and readiness of patients for inpatient rehabilitation at Epworth standalone rehabilitation facilities, versus patients more suitable for co-located acute haematology services and rehabilitation unit at Epworth Richmond.
Epworth Richmond offers an integrated inpatient haematology-rehabilitation service to those who are unable to participate in outpatient-based rehabilitation programs due to factors such as ongoing medical and nursing support needs, being significantly deconditioned, living in rural or remote locations, and ongoing cancer-related treatment plans. This particular inpatient program delivers both medical care and rehabilitation interventions including exercise, functional retraining, nutritional supplementation, and supportive counselling following acute cancer treatment or treatment of cancer related complications. A joint effort between acute haematology and rehabilitation teams aims to provide more efficient collaborative care and coordination of chemotherapy and radiation therapy treatment plans, monitoring of treatment-related response and management of any side-effects.
With outpatient-based rehabilitation programs, breast and general oncology outpatient rehabilitation programs are available at Epworth Hawthorn and general oncology outpatient rehabilitation programs at Epworth Camberwell, Epworth Brighton, and Epworth Geelong. The “Enhance” program, which is specifically designed for breast cancer patients, is an eight-week oncology program with exercise and education components. A multi-disciplinary team is involved including a physiotherapist, exercise physiologist, psychologist, occupational therapist, dietician, social worker, breast care nurse, and rehabilitation physician. This team provide comprehensive assessment of breast cancer patients and management of their functional impairments. Whilst this is a group-based service and patients vary in age, diagnosis, treatment and experiences, additional individual therapy is available where required to maximise functional outcomes for those patients.
With an ageing population and increasing cancer incidence, the need for rehabilitation services for cancer patients continues to increase. At Epworth Healthcare, the aim is to expand the ‘in-reach’ service across more acute cancer streams and to further develop inpatient and outpatient rehabilitation services and programs to meet the heterogenous needs of cancer patients.
Dr Sarah Kofoed & Dr Woo-Jin (Jeena) Kim
Rehabilitation Physicians, FAFRM
Epworth Healthcare, VIC
A review of rehabilitation in lymphoma
This article is a summary of a recently published review1:
“Amatya B, Khan F, Lew TE, Dickinson M. Rehabilitation in patients with lymphoma: An overview of Systematic Reviews. J Rehabil Med. 2021; 53(3): jrm00163”
Lymphomas are malignant neoplasms of the haematopoietic system, with aberrant proliferation of mature lymphoid cells or their precursors.2 They are broadly classified into non-Hodgkin’s lymphoma (NHL, 90 per cent) or Hodgkin’s lymphoma (HL). The new World Health Organization classification stratifies lymphomas based on the cell of origin (B-cell, T-cell/natural killer-cell and HL) or clinical behaviour (aggressive or indolent). An estimated 590,000 new cases of lymphoma (3.2 per cent of all cancers) were diagnosed in 2018 worldwide and its incidence is escalating, with a projected increase to approximately 919,000 by 2040.3,4 Amongst haematological malignancies globally, NHL is a leading cause of death and estimated to cause over 248,000 deaths (2.6 per cent of all cancers) in 2018.3 The total global economic burden of lymphoma is unknown. Treatment and supportive care requirements are resource intensive, with significant financial implications for patients, families, and healthcare systems. Loss of productivity also occurs as a result of the disease, treatment-associated morbidity, and premature mortality.
Current advancements in treatments and cancer detection/diagnosis have improved survival rates for patients with lymphoma (PwL), with age-standardised five-year net survival in adults ranging from 40 to 70 per cent globally in 2010-2014.5 The incidence of NHL is associated with increasing age, improved supportive care and availability of reduced-intensity chemotherapy regimens (such as PEP-C, R-miniCHOP, R-CVP), which are critical for older patients. Lymphomas and their treatment can be associated with short and medium-term residual deficits (physical, cognitive, psychosocial, and behavioural impairments), activities of daily living (ADL) limitations and participation restrictions. Treatment procedures (such as radiation therapy, chemotherapy, and/or surgery) can cause side effects and complications such as neuropathy, cardiotoxicity, cachexia, fatigue, deconditioning and myopathy. Further, various adjustment issues, increased care needs, inability to drive and return to work, financial constraints, and marital stress are reported during the transitional period.
Rehabilitation plays an integral part of any cancer management and there is evidence suggesting the beneficial effects of comprehensive rehabilitation. There is, however, an unmet need in the cancer population whereby only a limited number of survivors receive appropriate rehabilitation. Many general cancer guidelines do not incorporate recommendations for specific rehabilitation interventions. Therefore, this recent review evaluated existing evidence from published systematic reviews for the effectiveness of rehabilitation strategies for improved function, impairments, and participation in PwL.
A multipronged approach was undertaken for the comprehensive literature search, which included a search of health science databases: Cochrane Library, PubMed, EMBASE, and CINAHL (from inception to 1 October 2020), bibliographies of pertinent articles, journals, and grey literature. The study selection process was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All relevant data was extracted using standard proforma based on Cochrane methodology. The Measurement Tool to Assess Systematic Reviews (AMSTAR-2) was used to critically appraise the selected reviews. The Grade of Recommendation, Assessment, Development and Evaluation (GRADE) tool assessed the quality of evidence for each outcome in terms of risk of bias, inconsistency, indirectness, imprecision (random error) and publication bias. The quality of evidence was classified as: ‘high-quality’, ‘moderate-quality’, ‘low-quality’ or ‘very low-quality’ based on the difference between the effect estimate and true effect.
Overall, 12 systematic reviews (n = 101 studies, 87132 PwL) fulfilled review inclusion criteria, evaluating three broad categories of rehabilitation interventions (physical modalities, nutrition, and complementary medicine). Most reviews were of moderate to low methodological quality. The findings suggested moderate-quality evidence for exercise programs for improved fatigue and sleep disturbance; low-quality evidence for exercise therapy alone and Qigong/Tai chi for improved symptoms and overall quality of life, and an inverse association between sunlight/ultraviolet radiation exposure on NHL incidence, low-quality evidence for beneficial effects of yoga for sleep disturbances, and inconclusive evidence for the association of physical activity and lymphoma risk.
Despite established guidelines, standardised protocols for acute management of PwL and specific guidelines on structured rehabilitation programs are yet to be published. There was significant heterogeneity amongst the included systematic reviews in terms of primary studies involved, lymphoma patients, intervention protocols, rehabilitation settings, and the outcomes measured. Many of the evaluated interventions were too broadly described; specifically exercise interventions, without sufficient details (optimal settings, type, intensity and duration of therapy, cost-effectiveness) to enable replication of these interventions. Participant characteristics were heterogeneous amongst the studies regarding characteristics of lymphoma (type, lesion location and area, time since lymphoma, other comorbidities, age) that resulted in variability of findings. Further, the primary trials within the included reviews varied in their description of control arms, assessment time points, length of follow-up and outcome measures used. Therefore, pooling data for quantitative analyses was not possible, and a best-evidence synthesis was described using qualitative analyses.
This is the first review to systematically evaluate evidence from published systematic reviews to determine the effectiveness of rehabilitation interventions in PwL that aim to assist and guide treating clinicians in choosing an appropriate treatment approach. Despite a range of rehabilitation modalities used in this patient population, high-quality evidence for many is sparse. Some beneficial effects of exercise programs were noted for fatigue, psychological symptoms, and quality of life. The existing gaps in research and practise identified a need to be addressed in future robust studies.
Dr Bhasker Amatya, MD MPH DMedSci & Professor Fary Khan, FAFRM
Royal Melbourne Hospital & Peter MacCallum Cancer Centre, VIC
References:
- Amatya B, Khan F, Lew TE, Dickinson M. Rehabilitation in patients with lymphoma: An overview of Systematic Reviews. J Rehabil Med 2021; 53: jrm00163.
- Mugnaini EN, Ghosh N. Lymphoma. Prim Care 2016; 43: 661-675.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424.
- International Agency for Research on Cancer. Global Cancer Observatory (GCO) 2020 [cited 2020 16 Sep]; Website: https://gco.iarc.fr/
- Allemani C, Matsuda T, Di Carlo, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023-1075.
Walking after spinal cord injury: Fact or fantasy?
To walk again - this is the ultimate goal that every person with spinal cord injury (SCI) yearns for. As clinicians working in spinal rehabilitation medicine, we are always asking the question: How far are we from finding a “cure” for SCI?
Most recently, I had the privilege of chatting with a special guest, Professor Simon Gandevia, who is pioneering the world’s first randomised controlled study on transcutaneous spinal cord stimulation and walking in SCI.
Professor Gandevia (MD PhD DSc FAA FRACP) is an internationally renowned clinical neurophysiologist and researcher in the area of human movement and motor impairments. He is also the Deputy Director and co-founder of Neuroscience Research Australia (NeuRA), a leading institute on brain and nervous system research.

Professor Simon Gandevia
Professor Gandevia, thank you so much for your time today. Can you tell us about your current research?
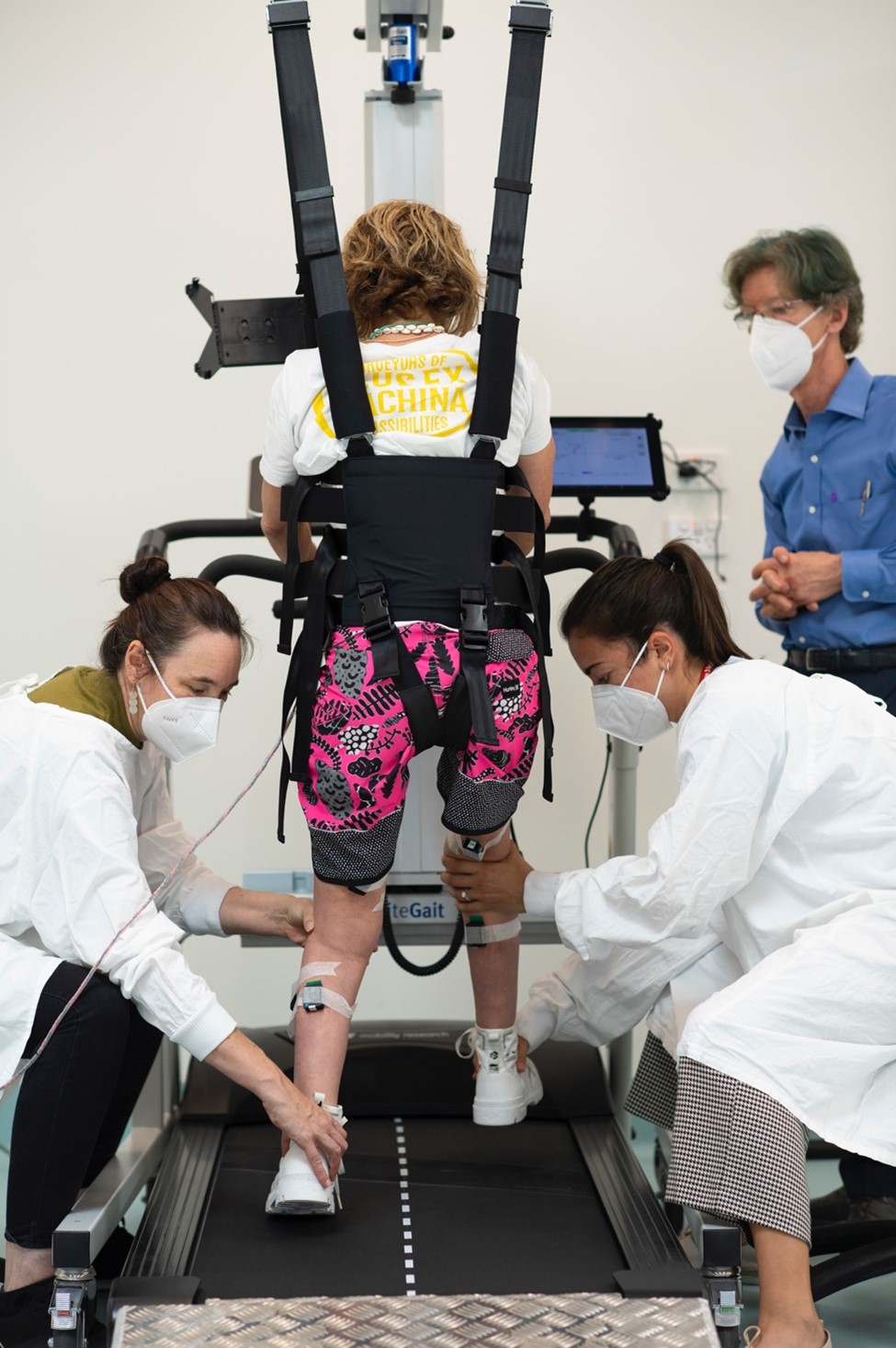
The primary new work going on at NeuRA is eWALK, a special trial involving transcutaneous (surface) spinal cord stimulation to see if this can help people with thoracic paraplegia to walk better. It is a chronic intervention lasting 12 weeks with stimulation sessions, three times a week. It’s a big commitment from the research team and even more so, from the participants.
e-WALK Trial
What makes your trial unique?
The critical thing is that our trial has a sham stimulus. So, it is a proper randomised controlled trial. And this is a world first. The researchers, the assessors, almost everyone has no idea whether the intervention is being delivered, or not delivered. And that is crucial to us.
Congratulations, that sounds like a real breakthrough! For those of us not involved in research, can you explain why randomised trials are so important?
You need proper trials; you don’t want to just hear dramatic case reports of something that may have caused the patient to move better. You’ll find that there are whole groups of people who are clearly desperate in favour of things working. So, you need proper research to find the rehab therapy that actually works. That is the focus for research spending.
Are you able to provide us a sneak preview, and tell us what your preliminary results are so far?
I can share with you that the trial is going surprisingly smoothly. There have been no unforeseen issues. This is good because it’s not trivial to move someone in and out of the gym, lifting them up in harnesses and using body weight support and treadmills.
Has COVID affected your trial?
It certainly changed how we did things. During this year, we needed appropriate protective gear for the therapists and participants. As in most medical research facilities, we insist on a high degree of vaccination coverage, so the risk for both the participants and staff is reduced. Surprisingly, in the midst of COVID, we got the trial up and running. We’re hoping to accelerate dramatically in January.
Well done! What other spinal cord injury research are you involved in?
We’re looking at different forms of training to help people with spinal cord injury cough better, because the likelihood of dying from pneumonia is about 150 times greater than that of the general population. There are other trials to see whether surface abdominal stimulation can enhance breathing, coughing, and bowel function in SCI. And some colleagues are doing work on virtual reality to see if that can enhance the sensory experience of people with functionally complete SCI.
Do you think there will ever be a “cure” for spinal cord injury?
Over my career, unfortunately, I’ve only seen ‘smallish’ improvements. I’m not an expert in cellular and various gene-based therapies. But it has been somewhat disappointing. There are many clues about therapies that may work in small animals, such as mice and rats. But it’s different translating that change into the much larger human spinal cord, where the circuitry may be more complex.
The therapy that appears to show most promise at the moment is some form of electrical stimulation. That is, activating the circuits that have survived and sending inputs to them, either from below or top down. The aim is to promote some degree of neuroplasticity and reprogramming to achieve some functional improvement.
Is it difficult to do SCI research in Australia?
It is extremely difficult to get rehabilitation type trials funded on the national stage for spinal cord injury. It is considered a niche area, not necessarily affecting a lot of people, but even a small improvement in a single patient’s function can make a huge difference to their life. A small gain can be amplified into a bigger effect. Spinal Cure Australia have been funding our current research.
Simon, what made you study medicine?
I got into medicine because I wanted to do something in science, and I came from a medical family. I also wanted to do something in law, but that wasn’t possible back then, which shows you how old I am! Halfway through medicine, I stopped and I did a PhD in physiology and went back into medicine. It exposed me to physiology, neurophysiology, and clinical medicine and I was able to blend these things together.
How did you get into the area of spinal cord injury research?
In my early days, when I was a PhD student and even as a medical student, I did research in Ward One at Prince Henry Hospital on people with spinal cord injury. So, my interest goes back a long way. And it’s been seriously reactivated in the last few years when we set up the Spinal Cord Injury Research Centre at NeuRA.
I just wanted to find out what you do in your spare time, if you have any extra time with everything you do!
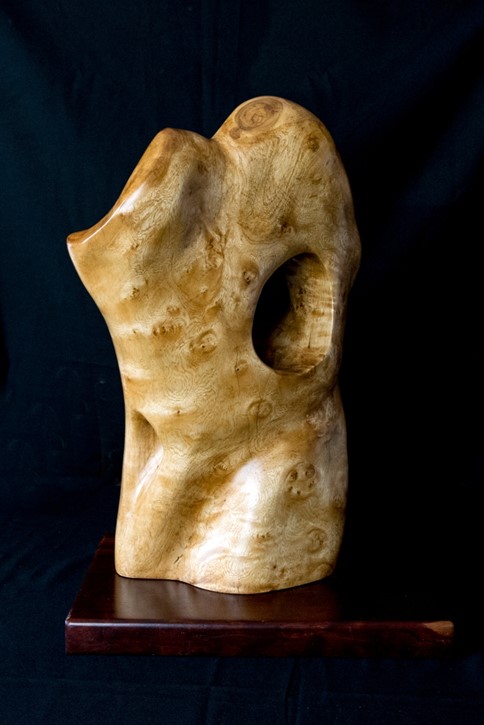
Yes, it is important to have spare time. I do a lot of things actually, but the easiest one to describe is I sculpt stone and wood. I started shortly after I graduated from medicine and I’ve kept it going. I’ve even had a sculpture exhibition and sold pieces. It is a creative outlet, and sometimes I have something pleasant at the end.
Creative outlets give you some time when you can lose yourself and think about other things. It can be helpful to thinking about a particular patient or a particular problem. All of that processing can be quietly going on while you are chiselling away at a bit of wood.
May we see one of your masterpieces?
Hardly masterpieces - this one I like is called “Torso”. It’s a little bit spinal-ish as well!
Simon Gandevia sculpture: “Torso”
Wow, this is amazing! It’s very beautiful and medically themed, I think I see a belly-button.
Simon, if people have questions about your work or trial, how can they best contact you?
Email for those interested in more information about the eWALK trial: ewalk@neura.edu.au
To view links to key studies that are currently underway, visit the Spinal Injury Research Centre at NeuRA.
Thank you so much for your time, Simon. You’ve given us such fascinating insight into your work and life. And thank you for all the research you do for our spinal patients. Both clinicians and patients appreciate it. We wish you the best of luck with your eWALK trial and look forward to the results.
Monica Ling FAFRM
Spinal Rehabilitation Medicine Specialist
Prince of Wales Hospital, NSW
New Zealand Spinal Cord Injury Registry: The journey thus far
Spinal Cord Injury (SCI) is rare, but complex. SCI can occur at any age and due to medical advancements, most people who have sustained SCI have near normal life expectancy. This brings progressive complexity. SCI management requires specialised multidisciplinary and coordinated approach of care from initial presentation through to life in the community. In Aotearoa New Zealand, adult acute care, rehabilitation, and follow up services for people with SCI are provided by the two supra-regional services. These are located in Christchurch under the Canterbury District Health Board (CDHB), and in Auckland under the Counties Manukau Health (CM Health).
Registries have been identified as key instruments in improving patient care and helping achieve optimal social, economic, and quality of life outcomes. Registries also assist health care planning, establish clinical research priorities, and allow international comparison of data. The need for a New Zealand SCI Registry (NZSCIR) was first identified in 1968. It was felt that a registry would be ideal to collect high-quality prospective data, with the potential to inform clinicians of the contributions of acute care, rehabilitation, and community interventions towards longer term patient outcomes. Various attempts to establish a registry in Aotearoa New Zealand have been unsuccessful in the past. Various researchers have published several challenges arising from the absence of a structured SCI registry.1
The NZ Spinal Cord Impairment Action Plan (2014-2019) was developed with the aim of achieving the best possible health and well-being outcomes for people who sustain SCI.2 Establishing an Aotearoa New Zealand SCI registry was a core objective of this action plan. A 12-month feasibility pilot study was undertaken in 2014 by CDHB, Accident Compensation Corporation (ACC), and Burwood Academy of Independent Living (BAIL) to investigate the implementation processes required to set up a SCI registry and provide the foundation for a business case. As part of the study, two registries were analysed and compared. These registries compared were The Rick Hansen SCI Registry (RHSCIR) and Victorian Spinal Injury Database. The RHSCIR was identified as an established, well-resourced national registry, with a high level of acute and surgical data, longitudinal follow up and having internationally standardised data sets and links with other international registries. This registry also provided the ability to partner with Rick Hansen Institute (RHI), with minimal cost for utilisation of a developed registry platform.3
NZSCIR was established in 2016 in partnership with the RHI, ACC, CM Health and CDHB, following the pilot study.1 It took eight months to develop and has been ongoing since 1 August 2016. An implementation plan was set up with RHI and protocols were developed including entry of non-traumatic SCI data (which was not part of the RHI data set) and adjusted to meet ethics requirements.3 A governance group was formed with representation from consumers, researchers, clinicians, funders, and managers. A registry coordinator at each site was established. Since inception, the NZSCIR publishes an annual report that is publicly available. 4
The most recent annual report is for the 2019 calendar year.4 Prior to establishment of NZSCIR, incidence of SCI in Aotearoa New Zealand was estimated at 30 per million, with approximately half being traumatic SCI. Based on NZSCIR 2019 annual data, the 2019 incidence was 45 per million (traumatic and non-traumatic SCI, inclusive of cauda equina) with 30 per million for traumatic SCI alone (Figure 1).
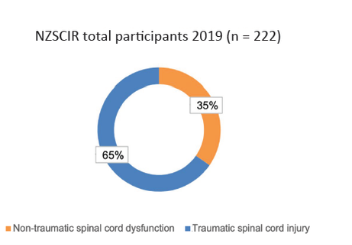
Figure 1: Percentage of traumatic and non-traumatic SCI
The leading cause of traumatic SCI in people aged 46 and above in 2019 is falls (Figure 2). The proportion of SCI from a fall increased from 17 per cent in the 0-30 age group to 82 per cent in the >76 years category. Transport followed falls as the next common cause of traumatic SCI, representing the leading cause of SCI in those under the age of 45 years. Most sport injuries included water sports (diving into pools or rivers).
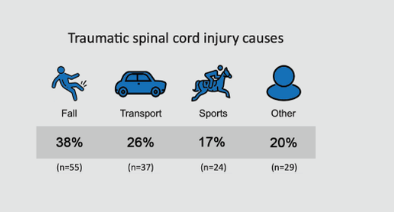
Figure 2: Causes of traumatic SCI
Vertebral column degenerative disorders were the most common cause of non-traumatic SCI (29 per cent) (Figure 3). Further non-traumatic SCI causes included malignant neoplasms (19 per cent), vascular disorders (17 per cent), infection (14 per cent), and other (21 per cent).
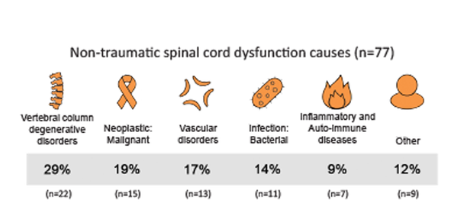
Figure 3: Causes of non-traumatic SCI
Using the Ministry of Health prioritised ethnicity reporting, across all participants, most were of European descent (59 per cent), followed by Māori (19 per cent) and Pacific people (17 per cent). Pacific people includes Samoan, Tongan, Niuean, and Cook Island Māori. People may choose more than one ethnicity and categories are not exclusive (Figure 4).
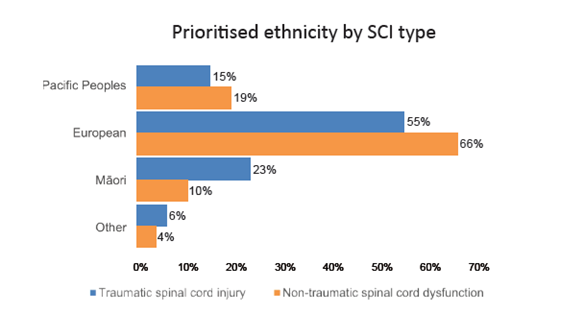
Figure 4: Ethnicity by SCI type
NZSCIR also captures length of stay (LOS) in acute and rehabilitation settings (Figures 5-8). The median LOS in 2019 was 16 days in acute care (down from 17 in 2018) and 55 days in rehabilitation (down from 63 in 2018). Those with tetraplegia spent more time in acute and rehabilitation settings (median 72 days) than those with paraplegia (59 days). Combined median LOS (not shown on graph) in rehabilitation for those with tetraplegia is down trending (2019 - 52 days; 2018 - 57 days; 2017 - 76 days).
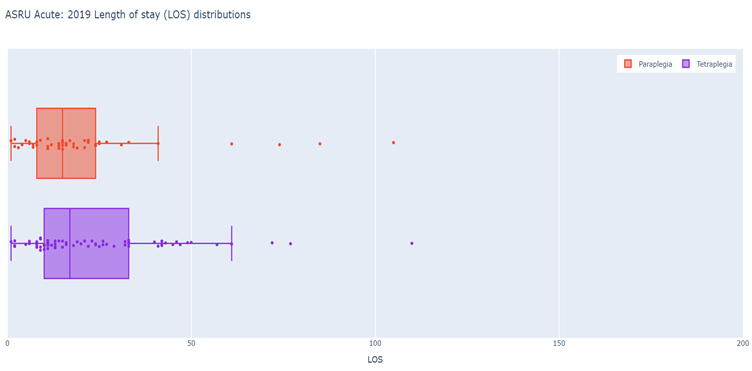
Figure 5: Auckland Acute - 2019 LOS (days) distributions
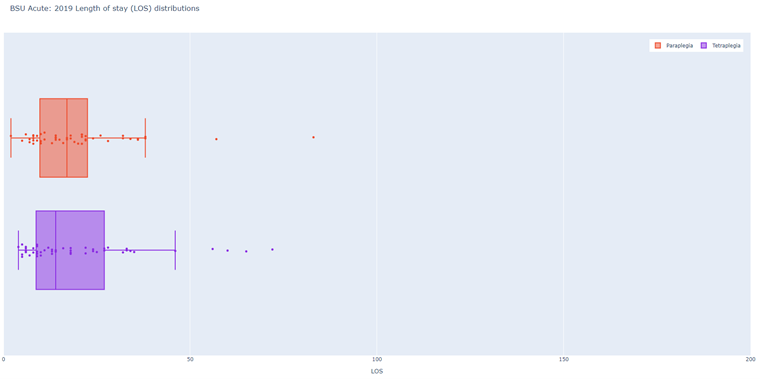
Figure 6: Christchurch Acute - 2019 LOS (days) distributions
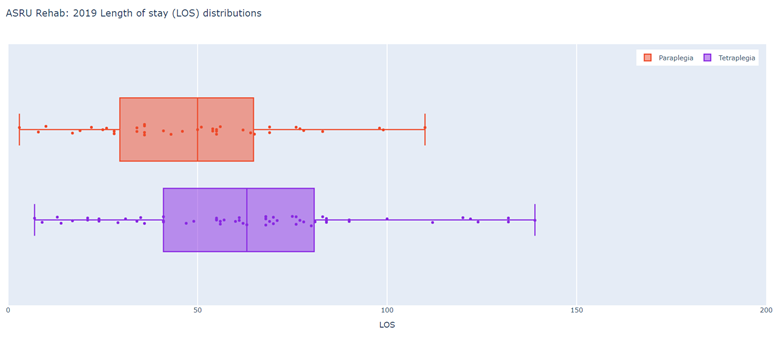
Figure 7: Auckland Rehabilitation - 2019 LOS (days) distributions
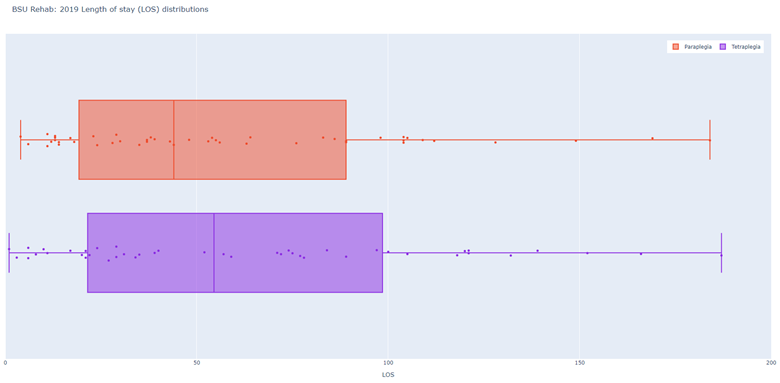
Figure 8: Christchurch Rehabilitation - 2019 LOS (days) distributions
The average age of NZSCIR participants was 51 years (Figure 9). Of those with traumatic SCI, the international trend of bimodal age distribution included the first peak occurring in young adults between 15 – 29 years, and the second peak occurring amongst older adults. Aotearoa New Zealand ’s second age peak occurs between 45 – 60 years, which is younger than international trend. The number of those with non-traumatic SCI tend to steadily increase with age, peaking at age 60 – 75 years.
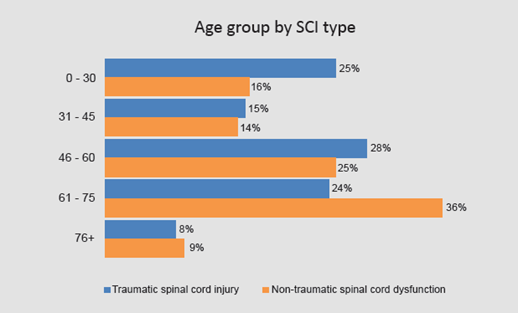
Figure 9: Age group by SCI type
The rates of surgery are higher in traumatic SCI compared to non-traumatic SCI (72 per cent vs. 66 per cent). On discharge, 36 per cent were independently walking in the community (able to walk 100 metres unsupervised with or without aids). Those with non-traumatic SCI were more likely to be walkers in the community (42 per cent vs. traumatic SCI 34 Per cent). Seventy per cent of discharges are to private residences in the community.
Secondary complications include pain, infections, and pressure injuries. On discharge, 72 per cent were receiving treatment for pain. Thirteen per cent of participants had urinary tract infections (UTIs) during their acute stay and 34 per cent during their rehabilitation phase. Respiratory complications occurred in 26 per cent of participants during the acute phase and 19 per cent during rehabilitation. Those with traumatic SCI were much more likely to have respiratory complications compared to those with non-traumatic SCI. Pressure injuries occurred in acute care in 12 per cent of cases and during rehabilitation phase in 22 per cent of cases.
NZSCIR has full Health and Disabilities Ethics Committee approval and report regularly to the Committee. The data access process is robust and requires researchers to undergo a rigorous request and review process before they gain access to de-identified data. Dashboard aggregate data can be collected for conference presentations, quality improvement activities within supra-regional spinal services, and service analyses. Clinicians have access to their own patients’ data for audits. Protocols can be assessed using NZSCIR data, such as the timing of traumatic SCI participants admitted to specialist centres and surgery.
Setting up and maintaining NZSCIR has not been without its challenges. Recruiting and consenting patients has been one of the challenges. Potential participants are approached for consent and if declined, a minimal data set is obtained as per the ethics approval. Consent rates have partially improved with increased allocation of workforce resources. Work is underway to assess the reasons why patients are declining the full data set for the registry. Issues with incomplete data entry were addressed by providing education and dedicated non-clinical time to clinicians for data entry. Securing long term funding for maintenance of registry is an ongoing challenge. Limited resources have delayed commencing and continuing with community data collection and entry. Follow-up of some participants has also been difficult. Despite these challenges, there are definite benefits of the NZSCIR in improving the understanding and management of SCI in Aotearoa New Zealand, whilst facilitating the translation of research into clinical practice. One of the key limitations of the NZSCIR is the absence of collection of any data for patients with SCI that have not been seen by either CM Health or CDHB supra-regional Spinal Services.
Dr Bensy Mathew, Dr Suresh Subramanian, Dr Dawn-Louise Adair
Auckland Spinal Rehabilitation Unit, New Zealand
We would like to acknowledge Tracey Croot (nzscir@cdhb.health.nz), BSU site NZSCIR coordinator and Jessica Ozumba (nzscir@middlemore.co.nz) ASRU NZSCIR site coordinator for their contributions to this article.
References:
- BSU SCI Registry Development Project (2015). Website: https://www.burwood.org.nz/bsu-sci-registry-development-project/
- New Zealand Spinal Cord Impairment Action Plan 2014-2019. Website: https://www.health.govt.nz/publication/new-zealand-spinal-cord-impairment-action-plan-2014-2019
- Howard-Brown C et.al. Establishment of the NZSCIR and its challenges. 2018 - Poster Presentation.
- New Zealand Spinal Trust. NZSCIR Annual Report 2019.
Ben's long and winding road to medicinal cannabis - Story by Ian Baker (Australian Quadriplegic Association)
(Reproduced with permission)
After years of awakening early and in pain, Ben Gruter swallowed some cannabis and slept for 10 hours. The former policy advisor to government now takes cannabis daily - and legally.
It has been 85 years since an American anti-marijuana propaganda film warned parents about the “Reefer Madness” that lay in wait for their children.
It has been nearly 30 years since former US President Bill Clinton denied he inhaled the marijuana that he had admitted to trying.
And it is a little more than five years since the Access to Medical Cannabis Act 2016 passed in Victoria, making it the first Australian state to legalise therapeutic use of the controversial drug. Other states have followed.
In Victoria, any doctor may apply to prescribe cannabis for any condition. However, it is not likely that your local GP will do so, and for three main reasons.
Firstly, almost no cannabis preparations have been registered as prescription drugs. Secondly, little is established about what ailments cannabis might help with, and how.
Thirdly, a common ingredient of medicinal cannabis, THC, is classed as a Schedule 8 poison and a drug of addiction like morphine, methadone, and amphetamine. A doctor must therefore seek authorisation before prescribing it for you and may perceive the approval process to be very onerous.
Nevertheless, a Senate inquiry was told early last year that more than 19,000 Australians had been prescribed medicinal cannabis, and there is evidence that the number has risen rapidly since.
Persistent pain
One such Australian is former academic and state government policy adviser Ben Gruter, 65, who has been living with paraplegia from an incomplete T5 spinal cord injury since 2012.
When he resolved to try medical cannabis, Ben had spent seven years experimenting with other treatments for his persistent neuropathic pain, which arrived with his injury. He experiences the pain as located in his right leg, extending from his hip to his foot.
“It’s pain caused by damage to the nerves,” explains Ben, who is a peer mentor with Australian Quadriplegic Association (AQA)/Spire.
“It feels like the most intense pins and needles you’ve ever felt. Some people describe it as burning.
“It’s constant from the moment I wake up until the time I go to bed. Stress will make it worse; being relaxed makes it better.”
Surveys indicate that more than half of people living with spinal cord injury live with neuropathic pain.
Prior treatments
Ben spent 16 months in treatment and rehabilitation after his injury, which arose from a bleed in his spinal column. Initially, he was given Panadol for his pain and when he sought more relief, he was prescribed an opioid, Oxynorm, and a pregabalin based anti-anxiety drug, Lyrica.
“By 12 months, it was pretty clear that not only weren’t these drugs working, but I was having terrible side effects,” Ben reported.
Under the care of a pain team at the Austin Hospital, he tried alternative opioids, three anti-depressants and ketamine, an anaesthetic. He said that his pain remained very troubling, except when ketamine put him to sleep.
Subsequently, Ben was treated by a multidisciplinary team at the hospital’s outpatient Pain Clinic, finding some relief in hydrotherapy and in the cognitive behavioural therapy offered by the team’s psychologist.
“Unfortunately, they could only have me as a patient for six months”, he explained, “because it’s a high demand service.”
Marital conflict
When he was injured, Ben had been married for just four and a half years, to Christine.
“The partner or other family members of someone with a spinal cord injury are also severely affected by it,” Christine observed.
“I think for me, the worst of it has been Ben’s pain. Seeing him in pain has just been horrific.
“And the effects of a lot of the medication he was given have been awful.”
Memory loss is a recognised side-effect of pregabalin. Christine says conflicts arose at home from Ben’s memory lapses.
“We would have a specific conversation about something and the next day, Ben would deny that we even discussed it,” she revealed. “I actually just thought he was being difficult.”
I know a friend
Ben said that he sought to reduce side-effects and cut his risk of addiction by decreasing his consumption of painkillers, in consultation with his GP.
For a few months, he took no drugs at all. Today, he takes no pregabalin and no Oxynorm, instead using an alternative opiate, Tapentadol, in a slow-release formulation.
“When Ben did go off all the drugs, it wasn’t living,” Christine said.
“The pain was so consuming; he just couldn’t do anything. He didn’t want to go out. He didn’t want to communicate with anyone. He couldn’t stand the grandkids coming in because they were noisy.”
It was Christine who suggested to Ben that he might try cannabis. The topic had come up in conversation with a friend whose husband also had a spinal cord injury.
“My friend said it had been helpful,” Christine recalled. “And her husband said it was helpful.
“So we met up and had a discussion, the four of us.”
Absolutely fantastic
“I’d seen the press reports,” says Ben, who like Christine had been exposed to the recreational use of cannabis when he was younger.
“Some medical practitioners said it’s very helpful. Other practitioners said it’s not helpful, and all the evidence isn’t in.
“I went into it with an open mind.”
At bedtime one evening, Ben used a dropper to administer himself a small dose of black-market cannabis oil, orally.
“For years, I’d been sleeping about three hours each night before I woke up in pain,” he said of the result.
“That first night when I took cannabis, I slept for 10 hours.
“It was absolutely fantastic.
“I got a little high and I got a dry throat. I didn’t get munchies.”
Christine added: “And he woke up like a human, instead of a pain unit.”
Obtaining medicinal cannabis
At the beginning of 2019, Ben sought a prescription from his GP for medicinal cannabis. He says his doctor told him he would help, but then concluded that the application for approval would be too burdensome. Instead, the GP wrote a referral to the specialist clinic that had treated Ben’s acquaintance.
Medicinal cannabis typically comes in two forms, only one of which contains THC - the component credited with producing euphoria. The active ingredient of the other form is CBD, which does not produce a high but is believed to have therapeutic value.
After interviewing Ben and reviewing his history, the clinic prescribed him CBD, advising him to increase the dose each week until it was effective.
“When I wasn’t getting a good enough effect, they added THC,” Ben reports.
The cannabis comes from a Melbourne compounding pharmacy in tablet-sized resin lozenges, by registered mail. The lozenges are packed in small plastic containers that carry Ben’s prescription details (the medication is available in other forms).
He takes half a CBD lozenge orally twice a day and then a THC lozenge at bedtime.
“My understanding is that a typical joint is about 10 milligrams of THC,” Ben said, referring to the recreational user’s cannabis-infused cigarette. “I take 6.25 milligrams of THC. So this is probably a little bit more than half a joint.
“On the dose that I take, I don’t get high. If I was taking a lot more, I would.
“It does make me drowsy - both the CBD and the THC make you drowsy. It tastes terrible. And I have a dry throat.
“I don’t know what would happen if I tried to really suppress the pain and take a lot more of it. I don’t want to try that, because I think I’d just fall asleep. So what’s the point of that?”
Hello Singapore!
From Christine’s perspective, the therapy has been transformative.
“I would say within two or three weeks, Ben was engaging,” she says. “He was engaging with me whereas he had been quite withdrawn before. He was engaging with the grandchildren. He was engaging with friends. We were going out. We were socialising.
“Within six weeks of him starting it, we had our first overseas holiday. Our first in seven years!”
The couple spent a week in Singapore, testing Ben’s capacity to make a flight twice as long to The Netherlands, where he was born.
“We had such a wonderful time. We were out every day. We couldn’t take the cannabis with us, but it took four days to wear off and then Ben still had his opioids.”
Prior to starting his cannabis therapy, Ben said: “I wouldn’t have dreamt of doing that trip.”
And he said the benefits have continued.
“The way I would describe it, is that it’s like the pain has been moved a bit away from you. So you are not focused on the pain anymore.
“The pain’s still there, perhaps level four rather than level six on a scale of 10, but it’s better. It’s not in your face.”
“And it’s not on his face,” Christine said. “He’s a lot more relaxed.”
A drug is a drug
Medicinal cannabis is not subsidised under the Pharmaceutical Benefits Scheme and Ben’s prescription costs the couple about $500 a month. That’s significant for them, and they’re aware it would be out of reach for some people.
They would like to see medicinal cannabis become available more cheaply and more readily. Ben would also welcome changes to traffic law.
Driving under the influence of THC is illegal and unlike alcohol, no threshold is specified.
“I’ve heard stories of people who won’t take THC because they’re afraid for their licence,” Ben said.
He added: “I take a very, very small dose, and that’s also worth saying. It is possible that I will need a higher dose in the future. But I don’t need it now.
“And I’ve got to say that although I’m a fan of cannabis - it’s probably the most benign of the drugs that I take for pain. It would be nice to get to a stage where I didn’t have any drugs and could just rely on physiotherapy and psychological pain management systems.”
Commentary by Dr Andrew Nunn – Medical Director of the Victorian Spinal Cord Service
Thank you Ben for your measured personal insight on your careful use of cannabis for pain following spinal cord impairment. Your personal account raises some issues for us all, and maybe very necessarily opens up this can of worms.
It is important that we do this, but it is also tricky. We need to develop a balanced view so that people with spinal cord injury, and those prescribing and funding the use of medicinal cannabis are well informed. The legislation on medicinal cannabis seems to be ahead of the science and a spectrum of issues and views.
Here is some commentary on the topic from the Australian Pain Society, a multidisciplinary not-for-profit organisation that seeks optimal access to pain prevention and management for all people (date reviewed: 19 March 2021).
“Cannabis-derived products are now available for use with therapeutic intentions in Australia and New Zealand. By far the most common reason for their use is chronic pain, however there is a critical lack of evidence that it provides a consistent benefit for any type of chronic non-cancer pain. More than 90 per cent of Special Access Scheme – Category B (SAS-B) approvals have been for chronic pain of various types.
The evidence available is either unsupportive of using cannabinoid products in chronic non-cancer pain (CNCP) or is of such low quality that no valid scientific conclusion can be drawn. Cannabidiol-only [CBD] formulations have not been the subject of a published randomised controlled trial (RCT) for pain indications, yet they are the most prescribed type of product.
In addition, evidence of harms does exist, particularly in relation to sedative effects, interactions with other medications, and neuropsychiatric effects (for products which contain tetrahydrocannabinol [THC]).
Given the above, the clinical use of cannabinoid products cannot be ethically recommended outside a properly established and registered clinical trial environment until high-quality evidence for specific indications is published.”
I see in this cautious approach legitimate concern about the possibility we will create a new problem relevantly similar to the over-prescription of OxyContin and other opioids for pain management in the United States, if with less florid complications. So we must think broadly.
Our Spinal Cord Service explored evidence and trial protocols with the industry pre-COVID, and we hope to understand this better through starting a trial on therapeutic use for spasticity.
Here are 12 points that deserve our attention:
- There are well over 1000 cannabis strains available, plus now many plant hybrids.
- Production methods for therapeutic use are variable, with many derivatives for each plant depending on fractionation method and even synthetic THC available.
- Cannabis formulations usually combine CBD and THC, and these can be supplied in many different proportions.
- Physiology is complex. There are many receptors in every body system, hence multiple applications (pain, spasticity, sleep and so on).
- Cannabis can have many positive and negative effects, so a balance is needed.
- Smoking is clearly not the best way to administer and other options such as oral, nasal or skin absorption contribute variability in both absorption of the medication and duration of action.
- There are many types of spinal cord injury, so the optimal dose will be very individual and time of action variable, plus placebo effects must be considered.
- Cannabis would often be used in combination with other agents. As Ben has recognised, it is important that we look at eclectic approaches and resist the assumption that we can procure a “quick fix”.
- Commercial drug costs and marketing are becoming a major influence.
- Prescribing cannabis is tricky and is subject to laborious follow-up requirements.
- Use of medicinal cannabis has functional considerations for driving and work.
- Research trials show varied results and for limited number of participants.
I believe we need to bring fresh eyes and an open mind as we consider the many views that are out there as well.
Dr Andrew Nunn
Director Victorian Spinal Cord Service, Austin Health VIC
Rehabilitation and older people – Geriatric rehabilitation
AFRM has been an international leader through its syllabus in rehabilitation and older people for many years. The syllabus was developed about 20 years ago and has been refined on several occasions. It should be revised again because of the explosion of literature about frailty, and post COVID-19 rehabilitation for older people.
In Australasia, we generally use the inclusive term rehabilitation and older people. However, in many countries, the other label “geriatric rehabilitation” is used.
There is much interest in Europe in this topic. An interdisciplinary group including rehabilitation physicians, geriatricians, nursing home physicians and other health professions is working to define geriatric rehabilitation specifically. The definition that has been formulated is – geriatric rehabilitation is a multidimensional approach of diagnostic, therapeutic and rehabilitative interventions, with the purpose of optimising functional capacity and independence, promoting physical activity, and preserving social participation in people with frailty and disabilities.
In Australia, we have COVID-19, an ageing population, the Royal Commission into Aged Care Quality and Safety and the recent Australian Institute of Health and Welfare Report on the prevalence of dementia as current issues. All suggest that rehabilitation and older people is a critical topic.
Professor Ian Cameron, FAFRM
Hornsby Hospital, NSW
John Walsh Centre for Rehabilitation Research
Chair in Rehabilitation Medicine, The University of Sydney
Osteosarcopenia is a musculoskeletal syndrome characterised by concurrent low muscle mass and function (sarcopenia), and low bone mineral density (osteoporosis). This “hazardous duet” has shared common risk factors and biological pathways.1 Osteosarcopenia has a strong association with impaired physical performance and balance, increased risk of falls and fractures.
Osteoporosis
Osteoporosis is a microarchitectural loss of bone tissue leading to decreased density and bone fragility. The preferred method of testing is a dual-energy x-ray absorptiometry (DEXA) scan of the central skeleton to measure bone mineral density (BMD) of the lumbar spine and hip (T scores). The World Health Organization (WHO) criteria for diagnosis of osteopenia and osteoporosis are T-scores of below -1 and below -2.5, respectively.
Risk factors for osteoporosis include older age, female gender, high alcohol intake, current smoking, obesity, menopause (females), low body weight, living in residential aged-care facilities, and low mobility and function. Peak bone mass is reached by the end of the third decade, and declines thereafter, more so in females. Between 24 and 49 million individuals over 50 years in North America, Europe, Japan, and Australia have osteoporosis.
The common consequences of osteoporosis are vertebral fractures and fragility fractures of the hip, wrist and humerus. Vertebral fractures are often clinically silent, with a higher risk of severe fracture in women than men over 70 years, but a higher risk of mild vertebral fracture in males. Fifty per cent of all low trauma fractures are non-hip and non-vertebral. All result in an increased risk of subsequent fracture, and all are associated with an increased risk of premature mortality.
With ageing, there is an imbalance between bone resorption and deposition, leading to accelerated loss of bone mass, and significant marrow fat infiltration.2 Women lose almost 50 per cent of trabecular bone and 30 per cent of cortical bone mass with oestrogen depletion following menopause, whilst men lose 30 per cent of bone mass during their lifetime.
Sarcopenia
The term sarcopenia is derived from the Greek: sarx = flesh and penia = loss, and refers to loss of muscle mass associated with ageing. However, sarcopenia is not only a disease of ageing, but shares common mechanisms with sarcopenia in chronic diseases (e.g., COPD, cancer) and endocrine diseases (e.g., diabetes, thyroid disease).3 This condition was recognised with separate ICD-10-CM code. Sarcopenia should not be defined solely on loss of muscle mass. Although muscle weakness is an inevitable consequence of sarcopenia, skeletal muscle becomes intrinsically weaker in old age, and the relationship between age-related reduction of muscle mass and strength is often independent of body mass (e.g., sarcopenic obesity).
The prevalence of sarcopenia increases with age; it is present in 10 per cent of people aged 60-70 years, and 30 per cent of those aged 80+ years. Sarcopenia has negative health consequences with a fourfold higher risk of mortality in people aged 79+ years, a threefold higher risk of functional disability, a significant association with risk of falling, a higher risk of hospitalisation, and prolonged hospitalisation.
Contributing factors to sarcopenia are changes in the nervous system and in muscle. With ageing, a loss of spinal motor neurons due to apoptosis results in a drastic reduction in muscle fibre numbers and size. In addition, there is a decline in neural organisation, an impaired capacity for reinnervation of denervated muscle fibres, and structural alterations in the neuromuscular junction. At the muscle level, there is a glycolytic-to-oxidative shift (i.e., a fast to slow twitch muscle transformation). This differs from the muscle changes associated with disuse atrophy, where there is a slow to fast twitch muscle transformation. Other features of sarcopenia are impaired muscle protein synthesis, impaired metabolic pathways, and fatty infiltration affecting mainly type II fibres.
Sarcopenia in spinal muscles is an independent predictor of vertebral compression fracture. Severe sarcopenia is associated with substantial increases in vertebral compression in the thoracic spine and shear loading along the whole spine.
Screening and assessment
Osteoporosis/osteopenia is diagnosed by DEXA. Fracture risk algorithms include the FRAX and QFracture (UK), the Garvan algorithm (Australia), and the CAROC (Canada).4 Whilst fragility fractures of the hip, wrist, and humerus have a clear clinical presentation, vertebral fractures are often silent. These may be identified through monitoring of height over time (a prospective height loss of >2cm), rib-pelvis distance (distance between costal margin and pelvic rim) for lumbar fractures, and occiput to wall distance for thoracic fractures.
The SARC-F tool5 is a screening tool for sarcopenia and clinical assessment should include measurement of handgrip strength, and the Short Physical Performance Battery (strength, function, balance). Muscle mass may be estimated using DEXA (reference standard), bioelectrical impedance (a surrogate measure), and CT or MRI.
Diagnostic criteria for sarcopenia vary among reference groups. The Consensus of the European Working Group on Sarcopenia in Older People6 recommends diagnosis based on documentation of criterion 1 plus criterion 2 or 3:
- Low muscle strength – probable sarcopenia
- Low muscle mass (appendicular skeletal muscle mass (kg)/height2 less than two SD below mean of reference group) – confirms diagnosis
- Low physical performance – severe sarcopenia if all criteria met
Management of osteoporosis and sarcopenia
Antiresorptive drugs such as bisphosphonates, denusomab or selective oestrogen receptor modulators are the first line approach for osteoporosis. However, fracture risk reduction with drugs is modest because the microstructural deterioration is not reversed. Anabolic agents (e.g., Teriparatide) and Romosozumab (humanised monoclonal antibody) enhance osteoblast activity.
There are no pharmacological treatments for sarcopenia. Non-pharmacological treatments include ensuring adequate calcium, vitamin D and protein intake, and limiting coffee and alcohol consumption and tobacco use. However, resistance exercise is a key evidence-based recommendation for management of osteosarcopenia and a number of trials have shown it to be beneficial in improving balance, bone health, and muscle.7,8
Professor Mary Galea AM PhD FAHMS
University of Melbourne, VIC
References:
- Crepaldi G, Maggi S. Sarcopenia and osteoporosis: A hazardous duet. J Endocrinol Invest 2005; 28(10 Suppl):66-68.
- Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 2017; 28:2781-2790.
- Borba VZC, Costa TL, Moreira CA, et al. Sarcopenia in endocrine and non-endocrine disorders. Eur J Endocrinol 2019; 180:R185-R199.
- Leslie WD, Lix LM. Comparison between various fracture risk assessment tools. Osteoporos Int 2014; 25:1-21.
- Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. JAMDA 2013; 14:531-532.
- Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48:16-31.
- Watson SL, Weeks BK, Weis L et al. High intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J Bone Mineral Res 2018; 33:211-220.
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; (3):CD002759.
January 2022
Editor introduction
This Rhaïa edition provides insight into cancer, spinal cord injury (SCI), and geriatric rehabilitation. Firstly, it is pleasing to see a lot more activity and growing interest amongst our colleagues, especially our younger Fellows, in the cancer rehabilitation space. Cancer patients are increasingly needing access to rehabilitation care and interventions. A recent exploratory pilot survey of rehabilitation colleagues working in cancer rehabilitation in Australia and Aotearoa New Zealand identified inadequate funding, lack of staff with expertise, and lack of collaboration between acute cancer care services and rehabilitation as major barriers towards successful implementation of cancer rehabilitation programs.1
The hope is to gradually dismantle these barriers, support a strategic approach to ensuring that cancer rehabilitation programs are available in our region, and develop sustainable, holistic cancer care models. Our cancer Special Interest Group (SIG), relevant working groups, and interested rehabilitation Fellows will play a vital role in promoting the awareness of cancer rehabilitation amongst acute specialty teams and community care providers. They will contribute to capacity building, establishing clinical guidelines, policies and pathways, encouraging research activities, and increasing advocacy of this area for the future.
With SCI rehabilitation, the latest SCI data from the Australian Spinal Cord Injury Register (ASCIR) showed 187 new cases of adult traumatic SCI in 2017-2018.2 Similar to previous findings, the majority were of younger age (25-34 years old), male gender, had neurological level of injury at C4 and incomplete tetraplegia (43 per cent) at discharge. Land transport crashes (46 per cent) represented the leading cause, followed by falls (36 per cent). For non-traumatic SCI (vascular disorders, degenerative conditions, cancer), out of 131 cases, males accounted for 60 per cent and the mean age was 58 years (range 19-89 years).
As we are aware, the short- and long-term disabilities faced by SCI patients are multiple. Useful resources can be found on SCI organisational websites (AQA Victoria, ParaQuad Tas, ParaQuad NSW, Spine & Limb Foundation WA, SCIA, SpinalCure, Spinal Life) and through societies such as ANZSCoS and ISCoS. Interesting research trials being undertaken include the eWalk (transcutaneous neurostimulation for SCI, Sydney) and robotic assisted game-based therapy.
We are also facing a population with increasing life expectancy. The need for ‘geriatric rehabilitation’ is growing in our region and so is the need for rehabilitation physicians with the essential skills to manage issues in older people. These can include sensory impairments (vision, hearing), falls, osteoporosis, malnutrition, depression, and cognitive impairment, including delirium and dementia. Professor Ian Cameron flags this area as a critical topic and Professor Mary Galea discusses osteosarcopenia.
I would like to express my utmost appreciation to all of our writers for their contribution to this edition of Rhaïa especially during a very busy and challenging year. If you have any interesting rehabilitation news that you would like to share with your colleagues, please let us know and we can include it in future publications. I wish you all the very best and I am sure that we are all looking forward to new and exciting things ahead in 2022.
Dr Krystal Song
Editor
References:
- Song K, Amatya B, Khan F. Cancer rehabilitation in Australia and New Zealand: a pilot cross-sectional survey. The Journal of The International Society of Physical and Rehabilitation Medicine 2021; 4(3): 146-155.
- Australian Institute of Health and Welfare: Harrison J, O’Brien D, Pointer S. Spinal cord injury, Australia, 2017–18. Injury research and statistics series no. 136. Cat. no. INJCAT 219. 2021. Canberra: AIHW.
Readers, please note that the below articles were written several months ago. Given the fast changing circumstances of the COVID-19 pandemic, some information may have changed since the articles were originally written, particularly in relation to the COVID-19 situation in Victoria.
President’s Report
I congratulate Editor Krystal Song for drawing together three very important areas of rehabilitation practice: cancer rehabilitation, spinal cord injury, and geriatric rehabilitation in this edition of Rhaïa.
I hope all three areas are now seen as core business by rehab medicine departments, but it was not always thus. They remind us how things change. Spinal Injury units were first established many decades ago in response to the number of young patients who were catastrophically injured in accidents, and whose prognoses were dire without specialist knowledge and treatment. The demographics have changed greatly during my career from a predominantly young person’s injury to one which is now seen almost as frequently in a much older population, related to falls.
In the early part of my career, cancer treatment was not something that involved rehabilitation services much at all. Fortunately, this has changed in the past 20 years and much of the credit is due to passionate advocates such as Professor Andrew Cole, who has contributed to this edition. Geriatric rehabilitation remains a very important part of practice for many of us and an important interface between us and our geriatric medicine colleagues in many of our hospitals and community practices.
Our colleague, Dr David Bowers, passed away recently and unexpectedly in the full flight of his professional career. Many of you would have known him, a spinal injury rehabilitation physician at the Royal North Shore Hospital in Sydney. Fitting tributes to his care and compassion were expressed on behalf of his colleagues and patients at his funeral service which was, of course, greatly limited in scope due to COVID-19 restrictions. On behalf of the Faculty, we would like to convey our sincere condolences to his wife and children.
Work at the Faculty continues on many fronts in a COVID restricted way, just as you are experiencing in your own hospitals and practices. We continue to meet regularly at executive and council level but these are all virtual meetings now. The unpredictable nature of the pandemic continues to frustrate attempts to normalise events. My recent plan to attend the New Zealand Rehabilitation Association meeting during the travel bubble and when things were more settled evaporated very rapidly and now appears hopelessly optimistic in retrospect. You probably have started attending virtual international meetings as I have. Whilst I find the convenience of not having to travel very helpful, I have had to confront the reality that these sessions are generally live in the middle of the night here, which tends to be rather disorienting if not overpoweringly soporific.
All the Faculty Examinations for 2021 have concluded, including the delayed Victorian Module 2 Examination in Melbourne, which was held on October 10th. Clearly, the COVID situation in Sydney and Melbourne were of concern and there was a contingency plan to delay until November if needed. We have started preparations for our 2022 examinations. The landscape for next year is not clear yet, although with vaccination rates at the level that they are, we are hopeful that there may be more normality by that time. Meetings of the Examination Working Group, comprised of the Faculty Executive, Chair of Assessment Committee and College education staff have been a regular feature of the last 12 months.
We are progressing our plans for a greater academic presence by virtue of a permanent academic post on the Faculty Council, which is underway and will link to a permanent research committee of Council. We continue to meet regularly with the Australian Rehabilitation Outcomes Centre (AROC), represented by the Director of UOW Health Services Research Institute Kathy Eagar, newly appointed Medical Director of AROC Maria Crotty, and recently appointed Director of AROC Ross Clifton. AROC is currently undergoing a strategic review, seeking to use its reputation as the best health benchmarking system in Australasia to seek ways where that data can be used to influence health policy at a state and national level. We are currently still seeking a Chair of the Management Advisory Group to replace Dr John Estell whose term finishes soon.
The place of rehabilitation in COVID-19 continues to be a very important topic. Dr Susan Graham represents the Faculty on the College’s COVID-19 Expert Reference Group and was recently involved in organising webinars on Long COVID. We remain concerned at reports of reduction in rehabilitation services and resources throughout the country in response to the need to expand acute services, particularly in Sydney recently and in Melbourne last year. Our concern is that these resources are often not restored or alternative proposals seem to appear almost under the guise of COVID. The literature suggests that there will be significant need for rehabilitation services for so called Long COVID and we need to be mindful that this may be the opportunity to ensure that rehabilitation services are not allowed to be permanently reduced and marginalised.
The College launched the ROC (RACP online communities) with the official launch of the Faculty community in October. I urge all members to join the online community. We hope that it will provide an easy method of communication between members, which will avoid the previous intractable problems when lists of email addresses were not available. We hope to be able to set up much easier direct communication for Branch Committees and Special Interest Groups.
There are always vacancies occurring in Faculty Committees, all the way up to executive and we are constantly looking to identify the next generation of Fellows to take over the important roles at the head of many of our committees. We need long-term successors for the Chairs of the Faculty Assessment and Faculty Training Committees and the President-elect becomes available in May 2022. I ask that all New Fellows consider where you could help and where your interest may lie. Those who have been working hard on committees already are requested to seriously consider moving to the Chair of the committee if you can. Committee members are often in the best position to take over the chair as they have a good understanding of how the committee works. It is often more difficult for someone to step in as the Chair of a committee when they have not been involved within before.
Rural colleagues have recently raised the issue of lack of continuity of trainees working in rural services and the difficulties this raises for their services from time to time. The rural workforce is a very big issue at both government and College level. The maldistribution of our health workforce is well documented and is indeed part of the problem with Indigenous health. Dr Jeremy Christley is the Faculty representative on the College’s Regional and Rural Physician Working Group. Rural training is a complex issue and one which the Faculty considered formally about 10 years ago, although the focus was about attracting Fellows to work in the country.
Trainees who have worked in the country are more likely to consider a rural career as a realistic option. I have had recent talks with the Australian College of Rural and Remote Medicine and the Rural Health Alliance both of whom understand the issue of convincing health professionals to see a rural career as a realistic medium- or long-term option. This is a complex issue and needs consideration of both the projected long-term rural workforce, the quality of the training experience, and the burden, both socially and financially, on trainees particularly now that many of our trainees are older than a generation ago and their life can be correspondingly more complicated. I am hopeful that if we can delineate and try to address the economic and social disincentives where they exist and simultaneously promote the value of rural training, this will allow us a non-controversial path to increase the number of trainees taking up country positions.
Please continue to make yourself aware of our College and Faculty prizes available every year. Please take the time to look at the College website and consider your colleagues who might be appropriate to nominate for these medals. There are several scholarships for those wishing to undertake research, with some specifically reserved for AFRM trainees and Fellows and others available to the wider College membership. Please promote these to your trainees and New Fellows who may be interested in a career in research.
A considerable amount of time was spent during the last two to three years developing materials to support an increase in the profile of rehabilitation medicine. These include PowerPoint presentations, brochures, and letters of introduction that can be used by Fellows to inform employers, area health service executives, and other health stakeholders of the value of rehabilitation medicine and its contribution to the health landscape. Unfortunately, much of this effort has been swamped by the pandemic that essentially sweeps all before it, but we have continued to meet with stakeholders to help them understand more about who we are and what we do. I have found these meetings to be beneficial, making personal links to partner organisations when the time is appropriate.
I continue to be very grateful for the support and assistance of my fellow Executive Members Jenny Mann (President-elect), Tim Geraghty (immediate past President), Caitlin Anderson (Chair, Faculty Education Committee), and Richard Seeman (Chair, Aotearoa New Zealand Branch), as well as our College staff, Jane Henderson (Executive Officer), Jo Goldrick (Faculties Manager) and Phil Munro (Peak Bodies Manager). I wish you all the very best in these trying times and we look forward to when the coming and expected COVID surges in New South Wales and Victorian hospitals have passed their peaks.
Dr Greg Bowring
AFRM President
Cancer rehabilitation
Until recently, cancer survivors have comprised only a small part of most rehabilitation physicians’ case load. Although some cancers have long been cured with early treatment, recent advances in chemotherapy, radiation therapy and immunotherapy now mean that treatment of quite advanced metastatic disease can lead to prolonged patient survival, for example with melanoma, breast, and prostate cancer. This, however, may be associated with significant impairment and functional disability. In this century, cancer has become another chronic disease, and survivors will respond to rehabilitation programs, just as others living with more ‘benign’ diseases do.
Rehabilitation helps cancer patients maximise their functional abilities and quality of life at each stage in their journey, as first recognised by Dietz in 1969. Programs start with preventive measures from the time of first diagnosis, later using restorative measures to regain premorbid functions lost with cancer treatment or combined with the effects of frailty in older cancer survivors. If significant physical problems are present (e.g., neurological or musculoskeletal impairments after treatment of cancer), intensive goal-orientated multidisciplinary programs are of great help. Finally, even in the later stages of the journey, relatively simple rehabilitation interventions provided jointly with palliative care clinicians can maximise residual mobility, activities of daily living or continence function, and greatly improve quality of life for both patients and their carers.1
Oncology care clinicians may not be aware of good outcomes from rehabilitation, may be focused on disease management rather than functional decline, or may wrongly think that if cancer-modifying treatment is of no further use, then rehabilitation also has little to offer. Likewise, cancer survivors and carers may assume and simply accept that progressive functional loss is inevitable with disease progression. All these are barriers to cancer survivors receiving appropriate access to rehabilitation.
Apart from major physical impairments, uncontrolled pain with depression, decreased activity and deconditioning are major causes of lost functional ability. It is important that baseline rest pain is properly controlled, with extra analgesics prescribed prior to general activities likely to provoke episodic pain (e.g., physiotherapy). Other activities that cause pain can be carefully deconstructed, to identify the specific motion or position that triggers pain, which can then be dealt with.
Both frailty and cancer are not uncommon in older people, and presence of frailty may well predate a cancer diagnosis. With early recognition, the older cancer survivor can be moved towards a non-frail state if given a combined program of nutritional support and physical exercise. This is especially important if there is complicating nausea or anorexia associated with chemotherapy or radiation therapy programs. At any age, if deconditioning is present in a cancer survivor, they should be offered an appropriate rehabilitation program for this, especially if their goals in life recovery include a return to work.
For cancer survivors with neurological deficits due to primary or metastatic brain or spinal cord lesions, and where the underlying malignant disease process has been treated and stabilised, there is good evidence that they respond to rehabilitation therapy with similar functional recovery, as do patients recovering from strokes or spinal cord ‘benign’ lesions, matched for anatomical location. This applies to therapy programs in individuals with speech and/or cognitive involvement, as well as with peripheral neurological involvement, whether from chemotherapy or individual peripheral nerve impingement by tumours.
It is particularly important that cancer survivors with brain stem disease are offered detailed speech therapy, occupational therapy, nutritional and swallowing programs, with appropriate dietary modifications or alternative feeding approaches, to avoid chest infections and frustrations with dysarthric speech and visual/coordination problems.
As with all rehabilitation, training and support of carers is most important, to maximise quality of life at home for the cancer survivor and their family/carers.
There is no place for therapeutic nihilism in the rehabilitation of a cancer survivor!
Associate Professor Andrew M Cole FAFRM
HammondCare Health, Greenwich Hospital
UNSW, School of Population Health NSW
References:
Cole AM. Medical rehabilitation and the palliative care patient. In: Cherny NI, Fallon MT, Kaasa S, Portenoy RK, Currow DC, editors. Oxford Textbook of Palliative Medicine. 6th ed. Oxford, England: Oxford University Press; 2021. P.255-264.
An update on cancer pain
Cancer rehabilitation is increasingly recognised as a crucial component of care for cancer survivors, with rehabilitation clinicians playing an integral role in supporting cancer patients during most phases of their cancer trajectory.1,2 Cancer pain is one of the most common symptoms experienced by cancer patients, with potential occurrence in any cancer type and at any stage from diagnosis to end-of-life care.3 Cancer pain can be classified as acute or chronic pain, and is defined as ‘pain that arises from cancer itself and/or treatment-related complication(s)’.4 If not managed well, cancer pain can have a significant impact on patients’ physical and psychological functions, resulting in difficulties carrying out their day-to-day activities and community roles.
Cancer pain is a complex condition that involves various pathophysiological mechanisms. Common cancer pain syndromes include chemotherapy-induced peripheral neuropathy (CIPN), pathophysiological fractures, widespread pain syndromes and persistent post-surgical pain.5 Contributing factors include nociceptive pain from surgery, radiation therapy, and cancer itself, from invasion of cancer into tissue or bone.
Peripheral and central sensitisation from persistent inflammation and repeated nociceptive input from cancer treatment and investigations may also contribute to persistent cancer pain.6 Many factors influence the experience of cancer pain including the patient’s background, experiences, cognitive status, and perpetuating biopsychosocial factors such as dealing with the notion of death, fear of cancer recurrence, and traumatic experiences from cancer treatments.7 Therefore, a thorough assessment of cancer pain is essential in understanding the mechanism of pain, patient factors, prognostic factors, and expectations of pain management to help guide appropriate treatment.
To date, there is limited evidence for the most effective treatment for cancer pain. Overall, a multidisciplinary approach to pain management is recommended due to the multi-faceted nature of cancer pain and associated symptoms that co-occur such as fatigue, anxiety and/or depression. Pharmacologically, opioids remain the mainstay of treatment for cancer pain but there is increasing awareness of the detrimental long-term effects of opioid use, especially in those with chronic pain syndromes. Side effects include constipation, cognitive impairment, endocrine disorders, and dependence.
Anti-neuropathic agents commonly used in cancer pain include anticonvulsants and antidepressants, but their efficacy is mixed and limited in evidence. Other adjuncts include steroids, alpha-agonists, and anti-inflammatories. Medicinal cannabis is emerging as an option but there is lack of evidence for its use in cancer pain, with implications of cost and tolerability in Australia.8 Non-pharmacological strategies and interventional techniques may be effective in opioid-unresponsive pain and should be considered to reduce polypharmacy and its effects. Nerve blocks, such as epidural injections, can be considered if there is an anatomical target or nociceptive driver. For those with opioid-unresponsive pain or side effects, an intrathecal opioid pump could be considered.
In conclusion, due to the complexity of cancer pain and its management, current evidence supports a more holistic approach to the management of cancer pain, with beneficial effects shown for rehabilitation programs incorporating multimodal interventions such as exercise and psychological strategies.
Dr Jamie Young MBBS, FAFRM, FFPMANZCA, Clin Dip Pall Care, AFRACMA, PhD
Pain & Rehabilitation Specialist
Peter MacCallum Cancer Centre VIC
References:
- Lisy K, Denehy L, Chan R, Khan F, Piper A, Jefford M. The state of cancer rehabilitation in Australia. Journal of Cancer Rehabilitation 2018; 1:9-13.
- Chowdhury RA, Brennan FP, Gardiner MD. Cancer rehabilitation and palliative care - Exploring the synergies. Journal of Pain and Symptom Management 2020; 60(6): 1239-1252.
- Van Den Beuken-Van MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. Journal of Pain and Symptom Management 2016; 51(6):1070-90.
- Yoong J, Poon P. Principles of cancer pain management: An overview and focus on pharmacological and interventional strategies. Australian Journal of General Practice 2018; 47(11):758-62.
- Caraceni A, Weinstein SM. Classification of cancer pain syndromes. Oncology 2001; 15(12):1627-40.
- Falk S, Bannister K, Dickenson AH. Cancer pain physiology. British Journal of Pain 2014; 8(4):154-62.
- Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. Journal of Pain and Symptom Management 2002. 24(5):526-42.
- Lintzeris N, Driels J, Elias N, Arnold JC, McGregor IS, Allsop DJ. Medicinal cannabis in Australia, 2016: the cannabis as medicine survey (CAMS‐16). Medical Journal of Australia 2018; 209(5):211-6.
Breast cancer rehabilitation: Current challenges
It is estimated that over 19,000 Australian women were diagnosed with breast cancer in 2020. This is a startling statistic, but encouragingly, over 90 per cent will survive at least five years.1 During my time seeing women for rehabilitation who have had a recent diagnosis of breast cancer, I have been in awe of their overwhelming positivity, future-thinking approach, and their remarkable ability to continue being mother, daughter, wife, friend and work colleague during what will be one of the most challenging and demanding times of their lives. For this very reason, breast cancer rehabilitation needs to be multi-faceted. It needs to assist women physically, emotionally, and practically to return to these roles which give their lives meaning and purpose.
The list of potential complications following breast cancer treatment is long. Physical effects, which are not necessarily any more or less burdensome than many of the emotional and psychological challenges, include lymphoedema, nerve palsies, chemotherapy-induced peripheral neuropathy, joint pains, fatigue, hot flushes, cording, adhesive capsulitis, osteoporosis, treatment related cardiac toxicity, and cancer-related fatigue. As rehabilitation physicians, we are uniquely placed to assist in the treatment of every single one of these issues. Yet, unless patients are referred to us, our skills go unused and patients miss out on prevention of and early intervention for cancer and treatment related complications.
As an example, a study by Younus et.al (2010) reported that 83 per cent of patients on aromatase inhibitors (AI) described joint-related pain, but only six per cent were referred to rehabilitation.2 Yet, the first line treatment for AI related joint pain is exercise. A breast cancer diagnosis should go hand-in-hand with a referral to rehabilitation, since increasing evidence is available for the benefits of exercise pre-surgery and whilst undergoing chemo- and radiation therapy treatment. At present, it is common for acute cancer care services to make a referral to rehabilitation once treatment is close to completion. However, a proactive approach to rehabilitation involving referral at time of diagnosis can help identify current issues and anticipate future impairments that may occur as a consequence of treatment or disease progression. Increasing awareness of the benefits of early rehabilitation amongst the community is also key; patients are powerful in initiating their own referrals. If patients knew that exercise improves survival and reduces all cause and breast cancer mortality, wouldn’t more patients ask their oncologists or breast surgeons for a rehabilitation referral? Surely the answer is, yes!
It is imperative that cancer rehabilitation programs are tailored to disease stage. A one-size-fits-all model for breast cancer fails to consider the wide-ranging differences in physical and psychosocial needs between those women with early-stage versus metastatic disease. Caution is required with exercise in those with metastatic bone disease due to risks of pathological fracture and spinal cord compression. MacMillan Cancer Support guidelines recommend that Mirel’s classification is used to determine relative fracture risk and therefore the potential need for prophylactic surgical treatment before exercise is undertaken.3
Mirel’s classification (Table 1) quantifies fracture risk based on site, pain, extent of cortical involvement and whether the lesion is lytic, blastic or mixed, and can be calculated from plain x-ray. Objective assessment of fracture risk allows exercise programs to be tailored to the individual patient to reduce potential complications and target safe exercises which still maximise physical outcomes. Activity for those with metastatic bone disease should avoid excessive torsion and reduce shear and compressive forces on affected skeletal sites. Metastases causing functional bone pain, especially in the lower limbs, may be suitable for prophylactic fixation prior to commencement of an exercise program (Table 2). Surgery for these patients has been shown to increase mobility and improve pain, thereby optimising function and improved quality of life.
Table 1: Mirel’s classification3
|
|
Score
|
|
|
1 point
|
2 points
|
3 points
|
|
Site
|
Upper limb
|
Lower limb
|
Peritrochanteric area of femur
|
|
Pain
|
Mild
|
Moderate
|
Functional impairment
|
|
Lesion
|
Blastic
|
Mixed
|
Lytic
|
|
Lesion size
|
<1/3 bone cortex
|
1/3 - 2/3 bone cortex
|
>2/3 bone cortex
|
Table 2: Mirel’s Clinical Recommendations3
|
Mirel’s Score
|
Clinical Recommendation
|
|
≤7
|
Radiotherapy and observation
|
|
8
|
Use clinical judgement
|
|
≥9
|
Prophylactic fixation
|
Further, there is a need to break down the socio-economic divide in provision of breast cancer rehabilitation. It is well documented that those with lower levels of education and income are less often referred to rehabilitation, have more advanced cancer at time of diagnosis, and have greater levels of comorbidity; all of which contribute to poorer outcomes.4 There needs to be improved accessibility to timely rehabilitation interventions for cancer patients across various cancer streams within the public health system in Australia and more funding dedicated to ‘prehab’ cancer services. Prehab for cancer patients can lessen the potential cardiotoxic effects of chemotherapy, improve tolerance to chemotherapy through systemic conditioning, prevent sarcopenia, and target specific body regions susceptible to disease or treatment-related dysfunction.
There is a long way to go before we can confidently say that there are adequate rehabilitation services for the over 70,000 women living with breast cancer in this country. However, the grass roots of current breast cancer rehabilitation programs demonstrate encouraging results and hopeful prospects for further growth. Let’s all raise awareness of our skill set in this area and continue to talk more about cancer rehabilitation, both within our specialty and outside of the rehabilitation sphere.
Dr Faye Jansen
Rehabilitation Physician, FAFRM
Cabrini Health Elsternwick, VIC
References:
- Australian Institute of Health and Welfare. Cancer data in Australia 2021 report. [Accessed 4/8/2021]. Website: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary
- Younus J, Kligman L. Management of aromatase inhibitor–induced arthralgia. Curr Oncol 2010; 17(1):87–90.
- Physical activity for people with metastatic bone disease: Guidance for healthcare professionals. Macmillan Cancer Support 2020. [Accessed 4/8/2021]. Website: https://www.macmillan.org.uk/healthcare-professionals/news-and-resources/guides/physical-activity-for-people-with-metastatic-bone-disease
- Bradshaw PT, Ibrahim JG, Khankari N, et al. Post-diagnosis physical activity and survival after breast cancer diagnosis: the Long Island Breast Cancer Study. Breast Cancer Res Treat 2014; 145:735–42.
Epworth Health cancer rehabilitation initiatives
Depending on cancer type, cancer effects and cancer-related treatments received, cancer patients may present with various rehabilitation issues including fatigue, pain, lymphoedema, chemotherapy induced peripheral neuropathy, steroid myopathy, deconditioning, and malnutrition. They often experience associated psychological distress and reduced overall quality of life. Cancer rehabilitation is increasingly recognised as a strategy to optimise the functional outcomes of cancer survivors from the time of diagnosis and throughout the trajectory of cancer continuum including treatment, survivorship, and advanced stages.
Current evidence supports cancer rehabilitation interventions in helping reduce symptom burden and improve quality of life amongst cancer patients. Evidence has also shown that exercise itself is beneficial in improving aerobic fitness, physical functioning, muscle strength, health-related quality of life, and reducing fatigue levels amongst patients with various cancer diagnoses.
Rehabilitation physicians play an important role in acute, subacute-inpatient, and subacute-outpatient care settings in being able to offer expertise in functional assessment and helping address impairments and activity limitations of cancer patients. At Epworth Healthcare in Victoria, a number of recent initiatives had been implemented to meet the rehabilitation needs of cancer patients across the care continuum. Of note, an ‘in-reach’ rehabilitation physician consult service was established this year within the acute haematology ward at Epworth Freemasons.
The rehabilitation physician assesses referred patients for physical and functional issues impacting on acute recovery and progress, working closely with acute therapists in setting achievable short-term goals. For patients who are not ready to participate in three hours of uninterrupted therapy per day (usual criteria for transfer to an inpatient rehabilitation unit) or are impacted by fatigue, this approach allows rehabilitation to commence in an acute setting for cancer patients. The rehabilitation physician also supports the nurse assessors in assessing suitability and readiness of patients for inpatient rehabilitation at Epworth standalone rehabilitation facilities, versus patients more suitable for co-located acute haematology services and rehabilitation unit at Epworth Richmond.
Epworth Richmond offers an integrated inpatient haematology-rehabilitation service to those who are unable to participate in outpatient-based rehabilitation programs due to factors such as ongoing medical and nursing support needs, being significantly deconditioned, living in rural or remote locations, and ongoing cancer-related treatment plans. This particular inpatient program delivers both medical care and rehabilitation interventions including exercise, functional retraining, nutritional supplementation, and supportive counselling following acute cancer treatment or treatment of cancer related complications. A joint effort between acute haematology and rehabilitation teams aims to provide more efficient collaborative care and coordination of chemotherapy and radiation therapy treatment plans, monitoring of treatment-related response and management of any side-effects.
With outpatient-based rehabilitation programs, breast and general oncology outpatient rehabilitation programs are available at Epworth Hawthorn and general oncology outpatient rehabilitation programs at Epworth Camberwell, Epworth Brighton, and Epworth Geelong. The “Enhance” program, which is specifically designed for breast cancer patients, is an eight-week oncology program with exercise and education components. A multi-disciplinary team is involved including a physiotherapist, exercise physiologist, psychologist, occupational therapist, dietician, social worker, breast care nurse, and rehabilitation physician. This team provide comprehensive assessment of breast cancer patients and management of their functional impairments. Whilst this is a group-based service and patients vary in age, diagnosis, treatment and experiences, additional individual therapy is available where required to maximise functional outcomes for those patients.
With an ageing population and increasing cancer incidence, the need for rehabilitation services for cancer patients continues to increase. At Epworth Healthcare, the aim is to expand the ‘in-reach’ service across more acute cancer streams and to further develop inpatient and outpatient rehabilitation services and programs to meet the heterogenous needs of cancer patients.
Dr Sarah Kofoed & Dr Woo-Jin (Jeena) Kim
Rehabilitation Physicians, FAFRM
Epworth Healthcare, VIC
A review of rehabilitation in lymphoma
This article is a summary of a recently published review1:
“Amatya B, Khan F, Lew TE, Dickinson M. Rehabilitation in patients with lymphoma: An overview of Systematic Reviews. J Rehabil Med. 2021; 53(3): jrm00163”
Lymphomas are malignant neoplasms of the haematopoietic system, with aberrant proliferation of mature lymphoid cells or their precursors.2 They are broadly classified into non-Hodgkin’s lymphoma (NHL, 90 per cent) or Hodgkin’s lymphoma (HL). The new World Health Organization classification stratifies lymphomas based on the cell of origin (B-cell, T-cell/natural killer-cell and HL) or clinical behaviour (aggressive or indolent). An estimated 590,000 new cases of lymphoma (3.2 per cent of all cancers) were diagnosed in 2018 worldwide and its incidence is escalating, with a projected increase to approximately 919,000 by 2040.3,4 Amongst haematological malignancies globally, NHL is a leading cause of death and estimated to cause over 248,000 deaths (2.6 per cent of all cancers) in 2018.3 The total global economic burden of lymphoma is unknown. Treatment and supportive care requirements are resource intensive, with significant financial implications for patients, families, and healthcare systems. Loss of productivity also occurs as a result of the disease, treatment-associated morbidity, and premature mortality.
Current advancements in treatments and cancer detection/diagnosis have improved survival rates for patients with lymphoma (PwL), with age-standardised five-year net survival in adults ranging from 40 to 70 per cent globally in 2010-2014.5 The incidence of NHL is associated with increasing age, improved supportive care and availability of reduced-intensity chemotherapy regimens (such as PEP-C, R-miniCHOP, R-CVP), which are critical for older patients. Lymphomas and their treatment can be associated with short and medium-term residual deficits (physical, cognitive, psychosocial, and behavioural impairments), activities of daily living (ADL) limitations and participation restrictions. Treatment procedures (such as radiation therapy, chemotherapy, and/or surgery) can cause side effects and complications such as neuropathy, cardiotoxicity, cachexia, fatigue, deconditioning and myopathy. Further, various adjustment issues, increased care needs, inability to drive and return to work, financial constraints, and marital stress are reported during the transitional period.
Rehabilitation plays an integral part of any cancer management and there is evidence suggesting the beneficial effects of comprehensive rehabilitation. There is, however, an unmet need in the cancer population whereby only a limited number of survivors receive appropriate rehabilitation. Many general cancer guidelines do not incorporate recommendations for specific rehabilitation interventions. Therefore, this recent review evaluated existing evidence from published systematic reviews for the effectiveness of rehabilitation strategies for improved function, impairments, and participation in PwL.
A multipronged approach was undertaken for the comprehensive literature search, which included a search of health science databases: Cochrane Library, PubMed, EMBASE, and CINAHL (from inception to 1 October 2020), bibliographies of pertinent articles, journals, and grey literature. The study selection process was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All relevant data was extracted using standard proforma based on Cochrane methodology. The Measurement Tool to Assess Systematic Reviews (AMSTAR-2) was used to critically appraise the selected reviews. The Grade of Recommendation, Assessment, Development and Evaluation (GRADE) tool assessed the quality of evidence for each outcome in terms of risk of bias, inconsistency, indirectness, imprecision (random error) and publication bias. The quality of evidence was classified as: ‘high-quality’, ‘moderate-quality’, ‘low-quality’ or ‘very low-quality’ based on the difference between the effect estimate and true effect.
Overall, 12 systematic reviews (n = 101 studies, 87132 PwL) fulfilled review inclusion criteria, evaluating three broad categories of rehabilitation interventions (physical modalities, nutrition, and complementary medicine). Most reviews were of moderate to low methodological quality. The findings suggested moderate-quality evidence for exercise programs for improved fatigue and sleep disturbance; low-quality evidence for exercise therapy alone and Qigong/Tai chi for improved symptoms and overall quality of life, and an inverse association between sunlight/ultraviolet radiation exposure on NHL incidence, low-quality evidence for beneficial effects of yoga for sleep disturbances, and inconclusive evidence for the association of physical activity and lymphoma risk.
Despite established guidelines, standardised protocols for acute management of PwL and specific guidelines on structured rehabilitation programs are yet to be published. There was significant heterogeneity amongst the included systematic reviews in terms of primary studies involved, lymphoma patients, intervention protocols, rehabilitation settings, and the outcomes measured. Many of the evaluated interventions were too broadly described; specifically exercise interventions, without sufficient details (optimal settings, type, intensity and duration of therapy, cost-effectiveness) to enable replication of these interventions. Participant characteristics were heterogeneous amongst the studies regarding characteristics of lymphoma (type, lesion location and area, time since lymphoma, other comorbidities, age) that resulted in variability of findings. Further, the primary trials within the included reviews varied in their description of control arms, assessment time points, length of follow-up and outcome measures used. Therefore, pooling data for quantitative analyses was not possible, and a best-evidence synthesis was described using qualitative analyses.
This is the first review to systematically evaluate evidence from published systematic reviews to determine the effectiveness of rehabilitation interventions in PwL that aim to assist and guide treating clinicians in choosing an appropriate treatment approach. Despite a range of rehabilitation modalities used in this patient population, high-quality evidence for many is sparse. Some beneficial effects of exercise programs were noted for fatigue, psychological symptoms, and quality of life. The existing gaps in research and practise identified a need to be addressed in future robust studies.
Dr Bhasker Amatya, MD MPH DMedSci & Professor Fary Khan, FAFRM
Royal Melbourne Hospital & Peter MacCallum Cancer Centre, VIC
References:
- Amatya B, Khan F, Lew TE, Dickinson M. Rehabilitation in patients with lymphoma: An overview of Systematic Reviews. J Rehabil Med 2021; 53: jrm00163.
- Mugnaini EN, Ghosh N. Lymphoma. Prim Care 2016; 43: 661-675.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424.
- International Agency for Research on Cancer. Global Cancer Observatory (GCO) 2020 [cited 2020 16 Sep]; Website: https://gco.iarc.fr/
- Allemani C, Matsuda T, Di Carlo, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023-1075.
Walking after spinal cord injury: Fact or fantasy?
To walk again - this is the ultimate goal that every person with spinal cord injury (SCI) yearns for. As clinicians working in spinal rehabilitation medicine, we are always asking the question: How far are we from finding a “cure” for SCI?
Most recently, I had the privilege of chatting with a special guest, Professor Simon Gandevia, who is pioneering the world’s first randomised controlled study on transcutaneous spinal cord stimulation and walking in SCI.
Professor Gandevia (MD PhD DSc FAA FRACP) is an internationally renowned clinical neurophysiologist and researcher in the area of human movement and motor impairments. He is also the Deputy Director and co-founder of Neuroscience Research Australia (NeuRA), a leading institute on brain and nervous system research.

Professor Simon Gandevia
Professor Gandevia, thank you so much for your time today. Can you tell us about your current research?
The primary new work going on at NeuRA is eWALK, a special trial involving transcutaneous (surface) spinal cord stimulation to see if this can help people with thoracic paraplegia to walk better. It is a chronic intervention lasting 12 weeks with stimulation sessions, three times a week. It’s a big commitment from the research team and even more so, from the participants.

e-WALK Trial
What makes your trial unique?
The critical thing is that our trial has a sham stimulus. So, it is a proper randomised controlled trial. And this is a world first. The researchers, the assessors, almost everyone has no idea whether the intervention is being delivered, or not delivered. And that is crucial to us.
Congratulations, that sounds like a real breakthrough! For those of us not involved in research, can you explain why randomised trials are so important?
You need proper trials; you don’t want to just hear dramatic case reports of something that may have caused the patient to move better. You’ll find that there are whole groups of people who are clearly desperate in favour of things working. So, you need proper research to find the rehab therapy that actually works. That is the focus for research spending.
Are you able to provide us a sneak preview, and tell us what your preliminary results are so far?
I can share with you that the trial is going surprisingly smoothly. There have been no unforeseen issues. This is good because it’s not trivial to move someone in and out of the gym, lifting them up in harnesses and using body weight support and treadmills.
Has COVID affected your trial?
It certainly changed how we did things. During this year, we needed appropriate protective gear for the therapists and participants. As in most medical research facilities, we insist on a high degree of vaccination coverage, so the risk for both the participants and staff is reduced. Surprisingly, in the midst of COVID, we got the trial up and running. We’re hoping to accelerate dramatically in January.
Well done! What other spinal cord injury research are you involved in?
We’re looking at different forms of training to help people with spinal cord injury cough better, because the likelihood of dying from pneumonia is about 150 times greater than that of the general population. There are other trials to see whether surface abdominal stimulation can enhance breathing, coughing, and bowel function in SCI. And some colleagues are doing work on virtual reality to see if that can enhance the sensory experience of people with functionally complete SCI.
Do you think there will ever be a “cure” for spinal cord injury?
Over my career, unfortunately, I’ve only seen ‘smallish’ improvements. I’m not an expert in cellular and various gene-based therapies. But it has been somewhat disappointing. There are many clues about therapies that may work in small animals, such as mice and rats. But it’s different translating that change into the much larger human spinal cord, where the circuitry may be more complex.
The therapy that appears to show most promise at the moment is some form of electrical stimulation. That is, activating the circuits that have survived and sending inputs to them, either from below or top down. The aim is to promote some degree of neuroplasticity and reprogramming to achieve some functional improvement.
Is it difficult to do SCI research in Australia?
It is extremely difficult to get rehabilitation type trials funded on the national stage for spinal cord injury. It is considered a niche area, not necessarily affecting a lot of people, but even a small improvement in a single patient’s function can make a huge difference to their life. A small gain can be amplified into a bigger effect. Spinal Cure Australia have been funding our current research.
Simon, what made you study medicine?
I got into medicine because I wanted to do something in science, and I came from a medical family. I also wanted to do something in law, but that wasn’t possible back then, which shows you how old I am! Halfway through medicine, I stopped and I did a PhD in physiology and went back into medicine. It exposed me to physiology, neurophysiology, and clinical medicine and I was able to blend these things together.
How did you get into the area of spinal cord injury research?
In my early days, when I was a PhD student and even as a medical student, I did research in Ward One at Prince Henry Hospital on people with spinal cord injury. So, my interest goes back a long way. And it’s been seriously reactivated in the last few years when we set up the Spinal Cord Injury Research Centre at NeuRA.
I just wanted to find out what you do in your spare time, if you have any extra time with everything you do!
Yes, it is important to have spare time. I do a lot of things actually, but the easiest one to describe is I sculpt stone and wood. I started shortly after I graduated from medicine and I’ve kept it going. I’ve even had a sculpture exhibition and sold pieces. It is a creative outlet, and sometimes I have something pleasant at the end.
Creative outlets give you some time when you can lose yourself and think about other things. It can be helpful to thinking about a particular patient or a particular problem. All of that processing can be quietly going on while you are chiselling away at a bit of wood.
May we see one of your masterpieces?
Hardly masterpieces - this one I like is called “Torso”. It’s a little bit spinal-ish as well!

Simon Gandevia sculpture: “Torso”
Wow, this is amazing! It’s very beautiful and medically themed, I think I see a belly-button.
Simon, if people have questions about your work or trial, how can they best contact you?
Email for those interested in more information about the eWALK trial: ewalk@neura.edu.au
To view links to key studies that are currently underway, visit the Spinal Injury Research Centre at NeuRA.
Thank you so much for your time, Simon. You’ve given us such fascinating insight into your work and life. And thank you for all the research you do for our spinal patients. Both clinicians and patients appreciate it. We wish you the best of luck with your eWALK trial and look forward to the results.
Monica Ling FAFRM
Spinal Rehabilitation Medicine Specialist
Prince of Wales Hospital, NSW
New Zealand Spinal Cord Injury Registry: The journey thus far
Spinal Cord Injury (SCI) is rare, but complex. SCI can occur at any age and due to medical advancements, most people who have sustained SCI have near normal life expectancy. This brings progressive complexity. SCI management requires specialised multidisciplinary and coordinated approach of care from initial presentation through to life in the community. In Aotearoa New Zealand adult acute care, rehabilitation, and follow up services for people with SCI are provided by the two supra-regional services. These are located in Christchurch under the Canterbury District Health Board (CDHB), and in Auckland under the Counties Manukau Health (CM Health).
Registries have been identified as key instruments in improving patient care and helping achieve optimal social, economic, and quality of life outcomes. Registries also assist health care planning, establish clinical research priorities, and allow international comparison of data. The need for a New Zealand SCI Registry (NZSCIR) was first identified in 1968. It was felt that a registry would be ideal to collect high-quality prospective data, with the potential to inform clinicians of the contributions of acute care, rehabilitation, and community interventions towards longer term patient outcomes. Various attempts to establish a registry in Aotearoa New Zealand have been unsuccessful in the past. Various researchers have published several challenges arising from the absence of a structured SCI registry.1
The NZ Spinal Cord Impairment Action Plan (2014-2019) was developed with the aim of achieving the best possible health and well-being outcomes for people who sustain SCI.2 Establishing an Aotearoa New Zealand SCI registry was a core objective of this action plan. A 12-month feasibility pilot study was undertaken in 2014 by CDHB, Accident Compensation Corporation (ACC), and Burwood Academy of Independent Living (BAIL) to investigate the implementation processes required to set up a SCI registry and provide the foundation for a business case. As part of the study, two registries were analysed and compared. These registries compared were The Rick Hansen SCI Registry (RHSCIR) and Victorian Spinal Injury Database. The RHSCIR was identified as an established, well-resourced national registry, with a high level of acute and surgical data, longitudinal follow up and having internationally standardised data sets and links with other international registries. This registry also provided the ability to partner with Rick Hansen Institute (RHI), with minimal cost for utilisation of a developed registry platform.3
NZSCIR was established in 2016 in partnership with the RHI, ACC, CM Health and CDHB, following the pilot study.1 It took eight months to develop and has been ongoing since 1 August 2016. An implementation plan was set up with RHI and protocols were developed including entry of non-traumatic SCI data (which was not part of the RHI data set) and adjusted to meet ethics requirements.3 A governance group was formed with representation from consumers, researchers, clinicians, funders, and managers. A registry coordinator at each site was established. Since inception, the NZSCIR publishes an annual report that is publicly available. 4
The most recent annual report is for the 2019 calendar year.4 Prior to establishment of NZSCIR, incidence of SCI in Aotearoa NZ was estimated at 30 per million, with approximately half being traumatic SCI. Based on NZSCIR 2019 annual data, the 2019 incidence was 45 per million (traumatic and non-traumatic SCI, inclusive of cauda equina) with 30 per million for traumatic SCI alone (Figure 1).

Figure 1: Percentage of traumatic and non-traumatic SCI
The leading cause of traumatic SCI in people aged 46 and above in 2019 is falls (Figure 2). The proportion of SCI from a fall increased from 17 per cent in the 0-30 age group to 82 per cent in the >76 years category. Transport followed falls as the next common cause of traumatic SCI, representing the leading cause of SCI in those under the age of 45 years. Most sport injuries included water sports (diving into pools or rivers).

Figure 2: Causes of traumatic SCI
Vertebral column degenerative disorders were the most common cause of non-traumatic SCI (29 per cent) (Figure 3). Further non-traumatic SCI causes included malignant neoplasms (19 per cent), vascular disorders (17 per cent), infection (14 per cent), and other (21 per cent).

Figure 3: Causes of non-traumatic SCI
Using the Ministry of Health prioritised ethnicity reporting, across all participants, most were of European descent (59 per cent), followed by Māori (19 per cent) and Pacific people (17 per cent). Pacific people includes Samoan, Tongan, Niuean, and Cook Island Māori. People may choose more than one ethnicity and categories are not exclusive (Figure 4).

Figure 4: Ethnicity by SCI type
NZSCIR also captures length of stay (LOS) in acute and rehabilitation settings (Figures 5-8). The median LOS in 2019 was 16 days in acute care (down from 17 in 2018) and 55 days in rehabilitation (down from 63 in 2018). Those with tetraplegia spent more time in acute and rehabilitation settings (median 72 days) than those with paraplegia (59 days). Combined median LOS (not shown on graph) in rehabilitation for those with tetraplegia is down trending (2019 - 52 days; 2018 - 57 days; 2017 - 76 days).

Figure 5: Auckland Acute - 2019 LOS (days) distributions

Figure 6: Christchurch Acute - 2019 LOS (days) distributions

Figure 7: Auckland Rehabilitation - 2019 LOS (days) distributions

Figure 8: Christchurch Rehabilitation - 2019 LOS (days) distributions
The average age of NZSCIR participants was 51 years (Figure 9). Of those with traumatic SCI, the international trend of bimodal age distribution included the first peak occurring in young adults between 15 – 29 years, and the second peak occurring amongst older adults. Aotearoa New Zealand second age peak occurs between 45 – 60 years, which is younger than international trend. The number of those with non-traumatic SCI tend to steadily increase with age, peaking at age 60 – 75 years.

Figure 9: Age group by SCI type
The rates of surgery are higher in traumatic SCI compared to non-traumatic SCI (72 per cent vs. 66 per cent). On discharge, 36 per cent were independently walking in the community (able to walk 100 metres unsupervised with or without aids). Those with non-traumatic SCI were more likely to be walkers in the community (42 per cent vs. traumatic SCI 34 Per cent). Seventy per cent of discharges are to private residences in the community.
Secondary complications include pain, infections, and pressure injuries. On discharge, 72 per cent were receiving treatment for pain. Thirteen per cent of participants had urinary tract infections (UTIs) during their acute stay and 34 per cent during their rehabilitation phase. Respiratory complications occurred in 26 per cent of participants during the acute phase and 19 per cent during rehabilitation. Those with traumatic SCI were much more likely to have respiratory complications compared to those with non-traumatic SCI. Pressure injuries occurred in acute care in 12 per cent of cases and during rehabilitation phase in 22 per cent of cases.
NZSCIR has full Health and Disabilities Ethics Committee approval and report regularly to the Committee. The data access process is robust and requires researchers to undergo a rigorous request and review process before they gain access to de-identified data. Dashboard aggregate data can be collected for conference presentations, quality improvement activities within supra-regional spinal services, and service analyses. Clinicians have access to their own patients’ data for audits. Protocols can be assessed using NZSCIR data, such as the timing of traumatic SCI participants admitted to specialist centres and surgery.
Setting up and maintaining NZSCIR has not been without its challenges. Recruiting and consenting patients has been one of the challenges. Potential participants are approached for consent and if declined, a minimal data set is obtained as per the ethics approval. Consent rates have partially improved with increased allocation of workforce resources. Work is underway to assess the reasons why patients are declining the full data set for the registry. Issues with incomplete data entry were addressed by providing education and dedicated non-clinical time to clinicians for data entry. Securing long term funding for maintenance of registry is an ongoing challenge. Limited resources have delayed commencing and continuing with community data collection and entry. Follow-up of some participants has also been difficult. Despite these challenges, there are definite benefits of the NZSCIR in improving the understanding and management of SCI in Aotearoa New Zealand, whilst facilitating the translation of research into clinical practice. One of the key limitations of the NZSCIR is the absence of collection of any data for patients with SCI that have not been seen by either CM Health or CDHB supra-regional Spinal Services.
Dr Bensy Mathew, Dr Suresh Subramanian, Dr Dawn-Louise Adair
Auckland Spinal Rehabilitation Unit, New Zealand
We would like to acknowledge Tracey Croot (nzscir@cdhb.health.nz), BSU site NZSCIR coordinator and Jessica Ozumba (nzscir@middlemore.co.nz) ASRU NZSCIR site coordinator for their contributions to this article.
References:
- BSU SCI Registry Development Project (2015). Website: https://www.burwood.org.nz/bsu-sci-registry-development-project/
- New Zealand Spinal Cord Impairment Action Plan 2014-2019. Website: https://www.health.govt.nz/publication/new-zealand-spinal-cord-impairment-action-plan-2014-2019
- Howard-Brown C et.al. Establishment of the NZSCIR and its challenges. 2018 - Poster Presentation.
- New Zealand Spinal Trust. NZSCIR Annual Report 2019.
Ben's long and winding road to medicinal cannabis - Story by Ian Baker (Australian Quadriplegic Association)
(Reproduced with permission)
After years of awakening early and in pain, Ben Gruter swallowed some cannabis and slept for 10 hours. The former policy advisor to government now takes cannabis daily - and legally.
It has been 85 years since an American anti-marijuana propaganda film warned parents about the “Reefer Madness” that lay in wait for their children.
It has been nearly 30 years since former US President Bill Clinton denied he inhaled the marijuana that he had admitted to trying.
And it is a little more than five years since the Access to Medical Cannabis Act 2016 passed in Victoria, making it the first Australian state to legalise therapeutic use of the controversial drug. Other states have followed.
In Victoria, any doctor may apply to prescribe cannabis for any condition. However, it is not likely that your local GP will do so, and for three main reasons.
Firstly, almost no cannabis preparations have been registered as prescription drugs. Secondly, little is established about what ailments cannabis might help with, and how.
Thirdly, a common ingredient of medicinal cannabis, THC, is classed as a Schedule 8 poison and a drug of addiction like morphine, methadone, and amphetamine. A doctor must therefore seek authorisation before prescribing it for you and may perceive the approval process to be very onerous.
Nevertheless, a Senate inquiry was told early last year that more than 19,000 Australians had been prescribed medicinal cannabis, and there is evidence that the number has risen rapidly since.
Persistent pain
One such Australian is former academic and state government policy adviser Ben Gruter, 65, who has been living with paraplegia from an incomplete T5 spinal cord injury since 2012.
When he resolved to try medical cannabis, Ben had spent seven years experimenting with other treatments for his persistent neuropathic pain, which arrived with his injury. He experiences the pain as located in his right leg, extending from his hip to his foot.
“It’s pain caused by damage to the nerves,” explains Ben, who is a peer mentor with Australian Quadriplegic Association (AQA)/Spire.
“It feels like the most intense pins and needles you’ve ever felt. Some people describe it as burning.
“It’s constant from the moment I wake up until the time I go to bed. Stress will make it worse; being relaxed makes it better.”
Surveys indicate that more than half of people living with spinal cord injury live with neuropathic pain.
Prior treatments
Ben spent 16 months in treatment and rehabilitation after his injury, which arose from a bleed in his spinal column. Initially, he was given Panadol for his pain and when he sought more relief, he was prescribed an opioid, Oxynorm, and a pregabalin based anti-anxiety drug, Lyrica.
“By 12 months, it was pretty clear that not only weren’t these drugs working, but I was having terrible side effects,” Ben reported.
Under the care of a pain team at the Austin Hospital, he tried alternative opioids, three anti-depressants and ketamine, an anaesthetic. He said that his pain remained very troubling, except when ketamine put him to sleep.
Subsequently, Ben was treated by a multidisciplinary team at the hospital’s outpatient Pain Clinic, finding some relief in hydrotherapy and in the cognitive behavioural therapy offered by the team’s psychologist.
“Unfortunately, they could only have me as a patient for six months”, he explained, “because it’s a high demand service.”
Marital conflict
When he was injured, Ben had been married for just four and a half years, to Christine.
“The partner or other family members of someone with a spinal cord injury are also severely affected by it,” Christine observed.
“I think for me, the worst of it has been Ben’s pain. Seeing him in pain has just been horrific.
“And the effects of a lot of the medication he was given have been awful.”
Memory loss is a recognised side-effect of pregabalin. Christine says conflicts arose at home from Ben’s memory lapses.
“We would have a specific conversation about something and the next day, Ben would deny that we even discussed it,” she revealed. “I actually just thought he was being difficult.”
I know a friend
Ben said that he sought to reduce side-effects and cut his risk of addiction by decreasing his consumption of painkillers, in consultation with his GP.
For a few months, he took no drugs at all. Today, he takes no pregabalin and no Oxynorm, instead using an alternative opiate, Tapentadol, in a slow-release formulation.
“When Ben did go off all the drugs, it wasn’t living,” Christine said.
“The pain was so consuming; he just couldn’t do anything. He didn’t want to go out. He didn’t want to communicate with anyone. He couldn’t stand the grandkids coming in because they were noisy.”
It was Christine who suggested to Ben that he might try cannabis. The topic had come up in conversation with a friend whose husband also had a spinal cord injury.
“My friend said it had been helpful,” Christine recalled. “And her husband said it was helpful.
“So we met up and had a discussion, the four of us.”
Absolutely fantastic
“I’d seen the press reports,” says Ben, who like Christine had been exposed to the recreational use of cannabis when he was younger.
“Some medical practitioners said it’s very helpful. Other practitioners said it’s not helpful, and all the evidence isn’t in.
“I went into it with an open mind.”
At bedtime one evening, Ben used a dropper to administer himself a small dose of black-market cannabis oil, orally.
“For years, I’d been sleeping about three hours each night before I woke up in pain,” he said of the result.
“That first night when I took cannabis, I slept for 10 hours.
“It was absolutely fantastic.
“I got a little high and I got a dry throat. I didn’t get munchies.”
Christine added: “And he woke up like a human, instead of a pain unit.”
Obtaining medicinal cannabis
At the beginning of 2019, Ben sought a prescription from his GP for medicinal cannabis. He says his doctor told him he would help, but then concluded that the application for approval would be too burdensome. Instead, the GP wrote a referral to the specialist clinic that had treated Ben’s acquaintance.
Medicinal cannabis typically comes in two forms, only one of which contains THC - the component credited with producing euphoria. The active ingredient of the other form is CBD, which does not produce a high but is believed to have therapeutic value.
After interviewing Ben and reviewing his history, the clinic prescribed him CBD, advising him to increase the dose each week until it was effective.
“When I wasn’t getting a good enough effect, they added THC,” Ben reports.
The cannabis comes from a Melbourne compounding pharmacy in tablet-sized resin lozenges, by registered mail. The lozenges are packed in small plastic containers that carry Ben’s prescription details (the medication is available in other forms).
He takes half a CBD lozenge orally twice a day and then a THC lozenge at bedtime.
“My understanding is that a typical joint is about 10 milligrams of THC,” Ben said, referring to the recreational user’s cannabis-infused cigarette. “I take 6.25 milligrams of THC. So this is probably a little bit more than half a joint.
“On the dose that I take, I don’t get high. If I was taking a lot more, I would.
“It does make me drowsy - both the CBD and the THC make you drowsy. It tastes terrible. And I have a dry throat.
“I don’t know what would happen if I tried to really suppress the pain and take a lot more of it. I don’t want to try that, because I think I’d just fall asleep. So what’s the point of that?”
Hello Singapore!
From Christine’s perspective, the therapy has been transformative.
“I would say within two or three weeks, Ben was engaging,” she says. “He was engaging with me whereas he had been quite withdrawn before. He was engaging with the grandchildren. He was engaging with friends. We were going out. We were socialising.
“Within six weeks of him starting it, we had our first overseas holiday. Our first in seven years!”
The couple spent a week in Singapore, testing Ben’s capacity to make a flight twice as long to The Netherlands, where he was born.
“We had such a wonderful time. We were out every day. We couldn’t take the cannabis with us, but it took four days to wear off and then Ben still had his opioids.”
Prior to starting his cannabis therapy, Ben said: “I wouldn’t have dreamt of doing that trip.”
And he said the benefits have continued.
“The way I would describe it, is that it’s like the pain has been moved a bit away from you. So you are not focused on the pain anymore.
“The pain’s still there, perhaps level four rather than level six on a scale of 10, but it’s better. It’s not in your face.”
“And it’s not on his face,” Christine said. “He’s a lot more relaxed.”
A drug is a drug
Medicinal cannabis is not subsidised under the Pharmaceutical Benefits Scheme and Ben’s prescription costs the couple about $500 a month. That’s significant for them, and they’re aware it would be out of reach for some people.
They would like to see medicinal cannabis become available more cheaply and more readily. Ben would also welcome changes to traffic law.
Driving under the influence of THC is illegal and unlike alcohol, no threshold is specified.
“I’ve heard stories of people who won’t take THC because they’re afraid for their licence,” Ben said.
He added: “I take a very, very small dose, and that’s also worth saying. It is possible that I will need a higher dose in the future. But I don’t need it now.
“And I’ve got to say that although I’m a fan of cannabis - it’s probably the most benign of the drugs that I take for pain. It would be nice to get to a stage where I didn’t have any drugs and could just rely on physiotherapy and psychological pain management systems.”
Commentary by Dr Andrew Nunn – Medical Director of the Victorian Spinal Cord Service
Thank you Ben for your measured personal insight on your careful use of cannabis for pain following spinal cord impairment. Your personal account raises some issues for us all, and maybe very necessarily opens up this can of worms.
It is important that we do this, but it is also tricky. We need to develop a balanced view so that people with spinal cord injury, and those prescribing and funding the use of medicinal cannabis are well informed. The legislation on medicinal cannabis seems to be ahead of the science and a spectrum of issues and views.
Here is some commentary on the topic from the Australian Pain Society, a multidisciplinary not-for-profit organisation that seeks optimal access to pain prevention and management for all people (date reviewed: 19 March 2021).
“Cannabis-derived products are now available for use with therapeutic intentions in Australia and New Zealand. By far the most common reason for their use is chronic pain, however there is a critical lack of evidence that it provides a consistent benefit for any type of chronic non-cancer pain. More than 90 per cent of Special Access Scheme – Category B (SAS-B) approvals have been for chronic pain of various types.
The evidence available is either unsupportive of using cannabinoid products in chronic non-cancer pain (CNCP) or is of such low quality that no valid scientific conclusion can be drawn. Cannabidiol-only [CBD] formulations have not been the subject of a published randomised controlled trial (RCT) for pain indications, yet they are the most prescribed type of product.
In addition, evidence of harms does exist, particularly in relation to sedative effects, interactions with other medications, and neuropsychiatric effects (for products which contain tetrahydrocannabinol [THC]).
Given the above, the clinical use of cannabinoid products cannot be ethically recommended outside a properly established and registered clinical trial environment until high-quality evidence for specific indications is published.”
I see in this cautious approach legitimate concern about the possibility we will create a new problem relevantly similar to the over-prescription of OxyContin and other opioids for pain management in the United States, if with less florid complications. So we must think broadly.
Our Spinal Cord Service explored evidence and trial protocols with the industry pre-COVID, and we hope to understand this better through starting a trial on therapeutic use for spasticity.
Here are 12 points that deserve our attention:
- There are well over 1000 cannabis strains available, plus now many plant hybrids.
- Production methods for therapeutic use are variable, with many derivatives for each plant depending on fractionation method and even synthetic THC available.
- Cannabis formulations usually combine CBD and THC, and these can be supplied in many different proportions.
- Physiology is complex. There are many receptors in every body system, hence multiple applications (pain, spasticity, sleep and so on).
- Cannabis can have many positive and negative effects, so a balance is needed.
- Smoking is clearly not the best way to administer and other options such as oral, nasal or skin absorption contribute variability in both absorption of the medication and duration of action.
- There are many types of spinal cord injury, so the optimal dose will be very individual and time of action variable, plus placebo effects must be considered.
- Cannabis would often be used in combination with other agents. As Ben has recognised, it is important that we look at eclectic approaches and resist the assumption that we can procure a “quick fix”.
- Commercial drug costs and marketing are becoming a major influence.
- Prescribing cannabis is tricky and is subject to laborious follow-up requirements.
- Use of medicinal cannabis has functional considerations for driving and work.
- Research trials show varied results and for limited number of participants.
I believe we need to bring fresh eyes and an open mind as we consider the many views that are out there as well.
Dr Andrew Nunn
Director Victorian Spinal Cord Service, Austin Health VIC
Rehabilitation and older people – Geriatric rehabilitation
AFRM has been an international leader through its syllabus in rehabilitation and older people for many years. The syllabus was developed about 20 years ago and has been refined on several occasions. It should be revised again because of the explosion of literature about frailty, and post COVID-19 rehabilitation for older people.
In Australasia, we generally use the inclusive term rehabilitation and older people. However, in many countries, the other label “geriatric rehabilitation” is used.
There is much interest in Europe in this topic. An interdisciplinary group including rehabilitation physicians, geriatricians, nursing home physicians and other health professions is working to define geriatric rehabilitation specifically. The definition that has been formulated is – geriatric rehabilitation is a multidimensional approach of diagnostic, therapeutic and rehabilitative interventions, with the purpose of optimising functional capacity and independence, promoting physical activity, and preserving social participation in people with frailty and disabilities.
In Australia, we have COVID-19, an ageing population, the Royal Commission into Aged Care Quality and Safety and the recent Australian Institute of Health and Welfare Report on the prevalence of dementia as current issues. All suggest that rehabilitation and older people is a critical topic.
Professor Ian Cameron, FAFRM
Hornsby Hospital, NSW
John Walsh Centre for Rehabilitation Research
Chair in Rehabilitation Medicine, The University of Sydney
Osteosarcopenia is a musculoskeletal syndrome characterised by concurrent low muscle mass and function (sarcopenia), and low bone mineral density (osteoporosis). This “hazardous duet” has shared common risk factors and biological pathways.1 Osteosarcopenia has a strong association with impaired physical performance and balance, increased risk of falls and fractures.
Osteoporosis
Osteoporosis is a microarchitectural loss of bone tissue leading to decreased density and bone fragility. The preferred method of testing is a dual-energy x-ray absorptiometry (DEXA) scan of the central skeleton to measure bone mineral density (BMD) of the lumbar spine and hip (T scores). The World Health Organization (WHO) criteria for diagnosis of osteopenia and osteoporosis are T-scores of below -1 and below -2.5, respectively.
Risk factors for osteoporosis include older age, female gender, high alcohol intake, current smoking, obesity, menopause (females), low body weight, living in residential aged-care facilities, and low mobility and function. Peak bone mass is reached by the end of the third decade, and declines thereafter, more so in females. Between 24 and 49 million individuals over 50 years in North America, Europe, Japan, and Australia have osteoporosis.
The common consequences of osteoporosis are vertebral fractures and fragility fractures of the hip, wrist and humerus. Vertebral fractures are often clinically silent, with a higher risk of severe fracture in women than men over 70 years, but a higher risk of mild vertebral fracture in males. Fifty per cent of all low trauma fractures are non-hip and non-vertebral. All result in an increased risk of subsequent fracture, and all are associated with an increased risk of premature mortality.
With ageing, there is an imbalance between bone resorption and deposition, leading to accelerated loss of bone mass, and significant marrow fat infiltration.2 Women lose almost 50 per cent of trabecular bone and 30 per cent of cortical bone mass with oestrogen depletion following menopause, whilst men lose 30 per cent of bone mass during their lifetime.
Sarcopenia
The term sarcopenia is derived from the Greek: sarx = flesh and penia = loss, and refers to loss of muscle mass associated with ageing. However, sarcopenia is not only a disease of ageing, but shares common mechanisms with sarcopenia in chronic diseases (e.g., COPD, cancer) and endocrine diseases (e.g., diabetes, thyroid disease).3 This condition was recognised with separate ICD-10-CM code. Sarcopenia should not be defined solely on loss of muscle mass. Although muscle weakness is an inevitable consequence of sarcopenia, skeletal muscle becomes intrinsically weaker in old age, and the relationship between age-related reduction of muscle mass and strength is often independent of body mass (e.g., sarcopenic obesity).
The prevalence of sarcopenia increases with age; it is present in 10 per cent of people aged 60-70 years, and 30 per cent of those aged 80+ years. Sarcopenia has negative health consequences with a fourfold higher risk of mortality in people aged 79+ years, a threefold higher risk of functional disability, a significant association with risk of falling, a higher risk of hospitalisation, and prolonged hospitalisation.
Contributing factors to sarcopenia are changes in the nervous system and in muscle. With ageing, a loss of spinal motor neurons due to apoptosis results in a drastic reduction in muscle fibre numbers and size. In addition, there is a decline in neural organisation, an impaired capacity for reinnervation of denervated muscle fibres, and structural alterations in the neuromuscular junction. At the muscle level, there is a glycolytic-to-oxidative shift (i.e., a fast to slow twitch muscle transformation). This differs from the muscle changes associated with disuse atrophy, where there is a slow to fast twitch muscle transformation. Other features of sarcopenia are impaired muscle protein synthesis, impaired metabolic pathways, and fatty infiltration affecting mainly type II fibres.
Sarcopenia in spinal muscles is an independent predictor of vertebral compression fracture. Severe sarcopenia is associated with substantial increases in vertebral compression in the thoracic spine and shear loading along the whole spine.
Screening and assessment
Osteoporosis/osteopenia is diagnosed by DEXA. Fracture risk algorithms include the FRAX and QFracture (UK), the Garvan algorithm (Australia), and the CAROC (Canada).4 Whilst fragility fractures of the hip, wrist, and humerus have a clear clinical presentation, vertebral fractures are often silent. These may be identified through monitoring of height over time (a prospective height loss of >2cm), rib-pelvis distance (distance between costal margin and pelvic rim) for lumbar fractures, and occiput to wall distance for thoracic fractures.
The SARC-F tool5 is a screening tool for sarcopenia and clinical assessment should include measurement of handgrip strength, and the Short Physical Performance Battery (strength, function, balance). Muscle mass may be estimated using DEXA (reference standard), bioelectrical impedance (a surrogate measure), and CT or MRI.
Diagnostic criteria for sarcopenia vary among reference groups. The Consensus of the European Working Group on Sarcopenia in Older People6 recommends diagnosis based on documentation of criterion 1 plus criterion 2 or 3:
- Low muscle strength – probable sarcopenia
- Low muscle mass (appendicular skeletal muscle mass (kg)/height2 less than two SD below mean of reference group) – confirms diagnosis
- Low physical performance – severe sarcopenia if all criteria met
Management of osteoporosis and sarcopenia
Antiresorptive drugs such as bisphosphonates, denusomab or selective oestrogen receptor modulators are the first line approach for osteoporosis. However, fracture risk reduction with drugs is modest because the microstructural deterioration is not reversed. Anabolic agents (e.g., Teriparatide) and Romosozumab (humanised monoclonal antibody) enhance osteoblast activity.
There are no pharmacological treatments for sarcopenia. Non-pharmacological treatments include ensuring adequate calcium, vitamin D and protein intake, and limiting coffee and alcohol consumption and tobacco use. However, resistance exercise is a key evidence-based recommendation for management of osteosarcopenia and a number of trials have shown it to be beneficial in improving balance, bone health, and muscle.7,8
Professor Mary Galea AM PhD FAHMS
University of Melbourne, VIC
References:
- Crepaldi G, Maggi S. Sarcopenia and osteoporosis: A hazardous duet. J Endocrinol Invest 2005; 28(10 Suppl):66-68.
- Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 2017; 28:2781-2790.
- Borba VZC, Costa TL, Moreira CA, et al. Sarcopenia in endocrine and non-endocrine disorders. Eur J Endocrinol 2019; 180:R185-R199.
- Leslie WD, Lix LM. Comparison between various fracture risk assessment tools. Osteoporos Int 2014; 25:1-21.
- Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. JAMDA 2013; 14:531-532.
- Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48:16-31.
- Watson SL, Weeks BK, Weis L et al. High intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J Bone Mineral Res 2018; 33:211-220.
- Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev 2009; (3):CD002759.